Have you ever considered instead of Phase 1 0 as starting point for your clinical trials? For a long time, Phase 1 drug development was the first step to get your drug approved for the market. Because of high R&D costs, long development times, and increased risk of failure, the FDA approved Phase 0 in 2006. What is Phase 0 and Phase 1 clinical trials? As a CRO (Clinical Research Organization) specializing in Phase 0 imaging studies, we will answer that question in this blog. The topic will be covered in general as a study design is different for every compound in drug development.
Book a meeting to discuss Phase 0 in your drug development process.
Book your meeting
Phase 0 vs Phase 1
Before we go over the “5 differences between Phase 1 0 in drug development” in depth, here is a quick overview that compares Phase 0 vs Phase 1, which are both early phase trials.
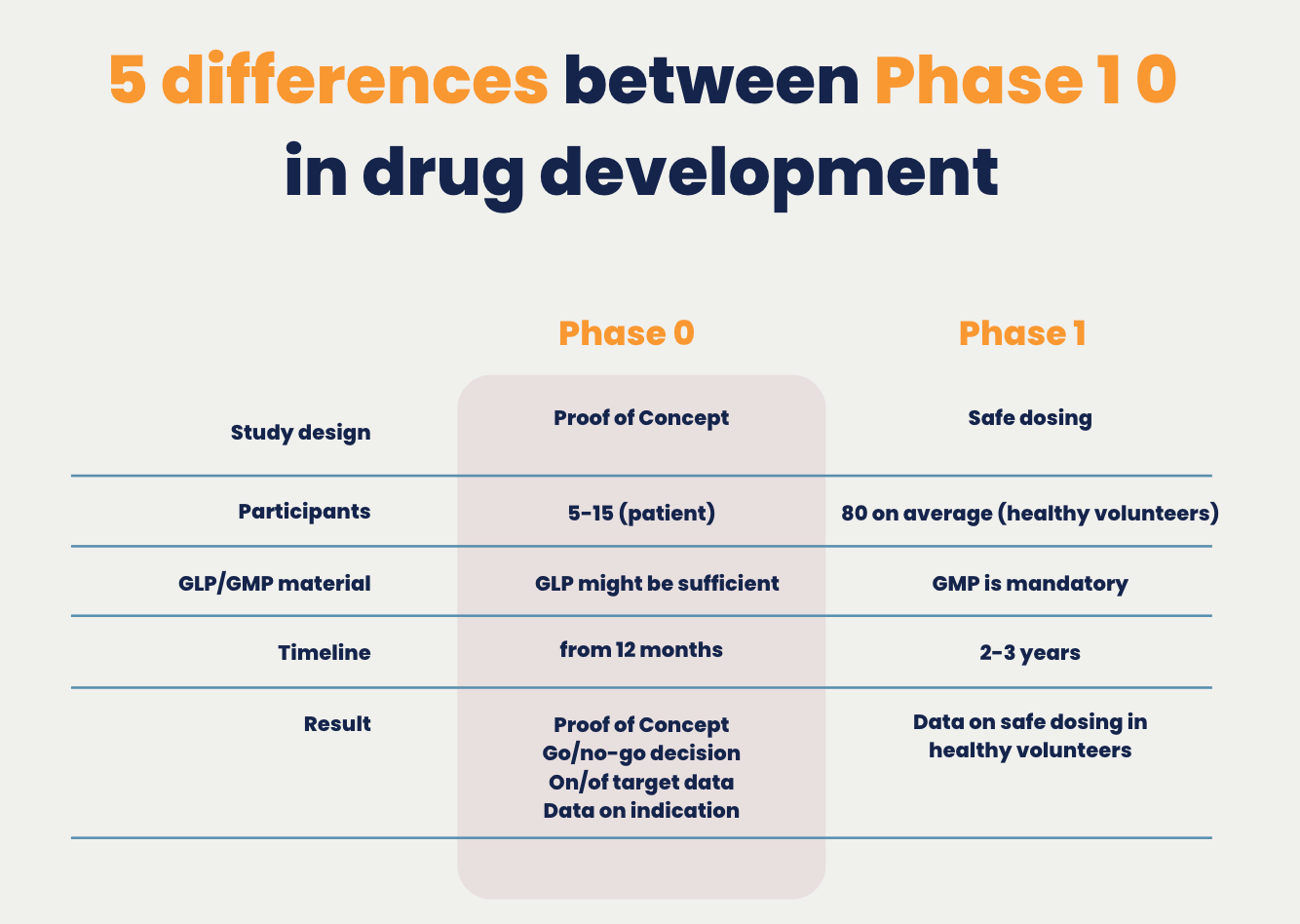
Comparison Phase 1 0
1. Phase 0 trials are specifically designed to speed up drug approval by providing a Proof of Concept. The study design of Phase 1, establishing safe dosing outlines in healthy volunteers, provides no Proof of Concept for your drug.
2. Phase 0 trials are conducted on your target population, while participants for Phase 1 trials are healthy volunteers that don’t express your targeted tissue.
3. To start a Phase 0 study GLP material is often sufficient, whereas for a Phase 1 clinical trial GMP material is mandatory.
4. Phase 0 can be completed about 3 times faster than a Phase 1 study and is by far the fastest of all clinical trials
5. A Phase 0 imaging study is FDA-approved and shows how a body processes a microdose of your drug. As a microdose has no therapeutic intent there is a lower risk of adverse side effects. In comparison, Phase 1 in drug development aims to find the best drug dose without checking the binding of the drugs to target tissue.
Phase 1 0 FAQ
Is Phase 0 the same as preclinical?
The early phase development of drugs is often referred to as preclinical development. The early phase in clinical trials, so in-human clinical trials, is often described as Phase 0. Although Phase 0 is an early phase clinical trial, it is not the same as a preclinical study. Namely, preclinical studies are, for example, cell studies (in vitro) and animal studies conducted in a laboratory. Their aim is to collect feasibility and safety data in animals. Meanwhile, Phase 0 looks at your drug’s PK/BD data in your target population. Under ICH M3 R2 guidance, exploratory in-patient studies can take place while still being in preclinical stage. When results from Phase 0 are positive, development continues into the early Phase 1 clinical trial.
What is a Phase 0 study?
A Phase 0 study is an in human study that differs significantly from the other stages of clinical trials. In this phase, you study the distribution of your drug in your target population. It helps drug developers to investigate if the drug targets the tissue of interest. The purpose of this study is to speed up the drug development process.
How the outcome of Phase 1 and 0 study is used differs. By administering the drug in patients and by imaging the uptake, valuable PK/BD data is gathered that can be used in future clinical trials. Phase 1 does not provide data on indication and uptake in target tissue since it can only be conducted on healthy volunteers.
What is the difference between Phase 0 and Phase 1 clinical trials?
We’ve answered the main questions about Phase 0 and 1 above. Now, we will discuss the differences between the two phases in terms of study design more in-depth.
1. The difference in design of early phase studies
There are traditionally 4 phases of clinical trials. Phase 0 and Phase 1 drug development are both early phase studies. In Phase 1, the main objective is to study toxicity and dosing. But it’s less useful to study dosing without having data on on-target and off-target uptake. The benefit of conducting before Phase 1 0: Phase 0 with imaging has the objective to study PK/BD data in patients. This data can be used to make a go/no-go decision before the next phase. In Phase 1 the PK/BD data from Phase 0 can point out where there is an increased chance of side effects.
Good to remember: Phase 1 clinical trial design differs from reality because there is no data from patients whereas Phase 0 gives in-patient data. Biodistribution and pharmacokinetics can be different between healthy volunteers and patients.
2. The difference in participants between Phase 1 0 early phase clinical trials
For a Phase 0 clinical trial, 5 to 15 participants are sufficient, while for Phase 1 you need around 80 participants. Fast inclusion is one of the reasons that make Phase 0 a faster in-human trial than Phase 1. If inclusion criteria are set correctly, finding the right participants shouldn’t be hard. TRACER has a broad network of medical institutions, giving you access to participants with many different indications, including rare indications.
If you are having problems with inclusion or questions on how your criteria affect the feasibility of your study, contact us.
3. GLP/GMP material needed: the difference between Phase 1 0
Early phase clinical trials need GLP (good laboratory practice) material. The difference between Phase 1 and 0 is the requirement of GMP (Good Manufacturing Practice) material. In Phase 0, GMP material is not required to start. In Phase 1 drug development, GMP material is mandatory which makes it more costly compared to the material used in Phase 0.
4. The differences in time between Phase 1 0 in early phase development
Early Phase 1 clinical trials take around 2 to 3 years on average to complete. Phase 0 takes around 12 months. With first patient in withing six to nine months.
5. The difference in results of phase 0 and phase 1 drug development
Based on the insights from Phase 0, you can make an informed go/no-go decision. The early development of clinical trials can be adjusted accordingly.
– Phase 0 results mainly focus on the distribution of the new medicine.
– Phase 1 clinical trials show the safety of the drug at several dose regimens and the pharmacokinetics of the drug.
What is Phase 1 and Phase 2?
Your Phase 0 data will especially come in use in Phase 2 of clinical trials. The difference between Phase 1/2 clinical trial is among others the type of participants. In Phase 1 of drug development, healthy volunteers participate in the study. In Phase 2, patients of the targeted group participate in the study. From your Phase 0 study you already have PK/BD data on different indications. Meaning, you can improve patient selection.
Do you want to know more about how a Phase 0 study can influence your Phase 1 trial? Continue reading about the use of Phase 0 data in Phase 1.
Or continue reading about molecular imaging in Phase 2 3 of clinical trials.
Schedule a meeting with TRACER to discuss in person how Phase 0 can benefit your drug development.
Book your meeting