What are the conditions to develop a drug under the FDA Accelerated Approval Program? Before we answer that question, it is important to know the basics of accelerated approval. First of all, what are the advantages and disadvantages of FDA Accelerated Approval? Furthermore, there are more FDA expedited programs available to speed up your drug development. Also, regulatory changes for exploratory trials allow drug developers to conduct in-patient trials prior to Phase 1. This is even applicable to drug development still in the preclinical stage.
Get a clear understanding of all possibilities regarding faster drug development.
Contact us
The need for expedited drug development
TRACER is a contract research organisation specializing in the acceleration of drug development. We utilize all available options regulators provide. Including the Accelerated Approval Program, exploratory trials (Phase 0), Proof of Concept trials (Phase 1b and 2a), and the possibilities under ICH M3 R2 guidance. We add imaging to clinical trials that may serve as secondary or even surrogate endpoints. All with the aim to bring potential life-changing and even life-saving therapies to patients faster. You can discuss all available options to accelerate the development of your drug, medical device, or diagnostic solution with TRACER.
Common benefits of the FDA Accelerated Approval Program
- Shorter trial duration.
- Reduced costs for clinical development.
- Faster market approval – about 3 years earlier compared to the clinical development time of a typical innovative drug) (Brown et al., 2022).
Disadvantages
- Drug developers are required to conduct confirmatory studies within a timeframe set by the FDA.
- The accelerated approval pathway FDA is currently under scrutiny due to the high prices and unproven clinical benefits of these drugs.
- Potential reform of FDA accelerated approval pathways and insurance coverage of these drugs has been initiated.
- Private insurance companies can already exclude drugs from the accelerated approval FDA list.
Accelerated approval requirements
In general, the requirement for therapies to enter expedited programs is that it is a therapy for a serious, severely debilitating, or life-threatening condition without satisfactory treatment options available. Therapeutic areas are versatile, however, oncology is with 83% of accelerated approvals between 2012–2021 most common. Another requirement is the justification of benefits over risks. Surrogate endpoints can be used when measuring clinical benefit takes too long or is too difficult to measure at all. These surrogate endpoints should have evidence to be a real predictor of clinical benefit.
General rules according to FDA guidance
Not only new therapies for an unmet need in serious or life-threatening diseases are considered. We list, according to FDA guidance, common criteria below:
- Diagnostic products that would improve clinical benefits.
- Therapies with an improved clinical outcome when used as a replacement of, or in addition to, existing therapy.
- Products targeting the same diseases where the only currently available options are also under accelerated approval with surrogate endpoints.
- New therapies that are compatible with existing, deemed critical, therapies. Only if currently available therapies are incompatible.
- Therapies for patients who do not respond to or cannot tolerate existing treatment.
- Products that prevent or ease serious side effects from the existing treatment or serve as an alternative therapy causing no or less severe serious adverse events. E.g. lower toxicity / drug-drug-interactions.
- Products that can prevent or slow the progression of a serious condition.
- Products that can fill an existing or anticipated shortage of existing therapies.
- Products related to an existing or emerging health issue (e.g., COVID-19).
Requirements for AA drug labeling
Since 2019 an FDA guidance document has been available for AA drug labeling. The label should state that the drug was approved via the FDA AA pathway and should include the surrogate endpoints and confirmatory study. A study by Ballreich et al., 2023 found that of 110 drug-indication pairs, 13% lacked information on the approval pathway, and 6% did not provide the surrogate endpoints.
The four expedited programs FDA
There are currently four FDA expedited programs. Application to these programs is not limited to one. FDA programs and their main features are listed below.
- Accelerated Approval (market approval based on surrogate endpoints)
- Breakthrough Therapy Designation (Intensive FDA guidance)
- Fast Track Designation (Intensive FDA guidance and fast FDA review)
- Priority Review Designation (Fast FDA review)
In this article, we focus on accelerated approval, but will briefly discuss the other programs at the end.
Surrogate endpoints vs clinical endpoint
Clinical endpoints are measurements of how a patient feels, functions, and if the patient survives. This is also defined as survival, cure, remission, or mortality, morbidity, and Health-Related Quality of Life (HRQoL).
With the FDA Accelerated Approval Program, surrogate endpoints in clinical trials could be sufficient for market approval. These surrogate endpoints should not be confused with secondary endpoints in clinical trials. A surrogate endpoint serves as a primary endpoint if clinical endpoints are difficult to measure or if the time to evaluate the endpoint is considered too long. Surrogate markers in clinical trials can only be used if there is evidence of correlation with and high predictability for true clinical endpoints. Acceptance for surrogate endpoints is done individually for each case.
Surrogate outcome examples
Surrogate outcomes in clinical trials should display a high level of prediction of a clinical endpoint.
An easy-to-understand example is tumor shrinkage, indicating the effect of the drug on cancer and predictor of a clinical benefit.
Another example, reduction in amyloid beta plaques, can predict improvement of cognitive function for patients with Alzheimer’s disease. However, studies show that examples of surrogate endpoints are not always valid. For instance, a study by Mozersky et al., 2022 suggests clinical benefit for Alzheimer’s disease has not yet been established based on amyloid reduction.
Surrogate endpoints list
A table of surrogate endpoints is available on the site of the FDA. This list of surrogate endpoints has been the result of the 21st Century Cures Act of 2016. The table is updated twice a year. Therefore, endpoints already used in clinical trials are listed, but others may be accepted.
Biomarkers and surrogate endpoints in clinical trials
What is the surrogate biomarker definition? A biomarker that predicts clinical benefit may serve as a surrogate endpoint. Evidence should be available for biomarkers and surrogate endpoints in relation to clinical benefit(s). As the surrogate endpoint examples underline, achieving a surrogate endpoint does not always lead to clinical benefit. If you want to learn more about surrogate endpoints in clinical trials definition and operational criteria, here are multiple resources.
FDA accelerated approval timeline
After you receive accelerated approval, your drug may enter the market. The FDA will set the timeline for the therapeutic confirmatory trials phase. Initiation of mandatory studies can already start before market entry. Check FDA Accelerated Approval lists to obtain a better understanding of confirmatory trials. You can find lists of AA drugs with clinical benefits approved, but also for AA drugs still in trials or withdrawn from the market. There are hundreds of studies in these lists. Contact TRACER to discuss what studies might be comparable and relevant to your study.
FDA accelerated approval confirmatory trial
When a surrogate end point is used, a post marketing confirmatory trial should still verify clinical benefit. If the confirmatory studies do not fully or sufficiently support the surrogate end points in clinical trials, the FDA reserves the right to withdraw approval or alter approved indications. Regular approval is granted if the confirmatory trial demonstrates clinical benefit and is therefore considered successful.
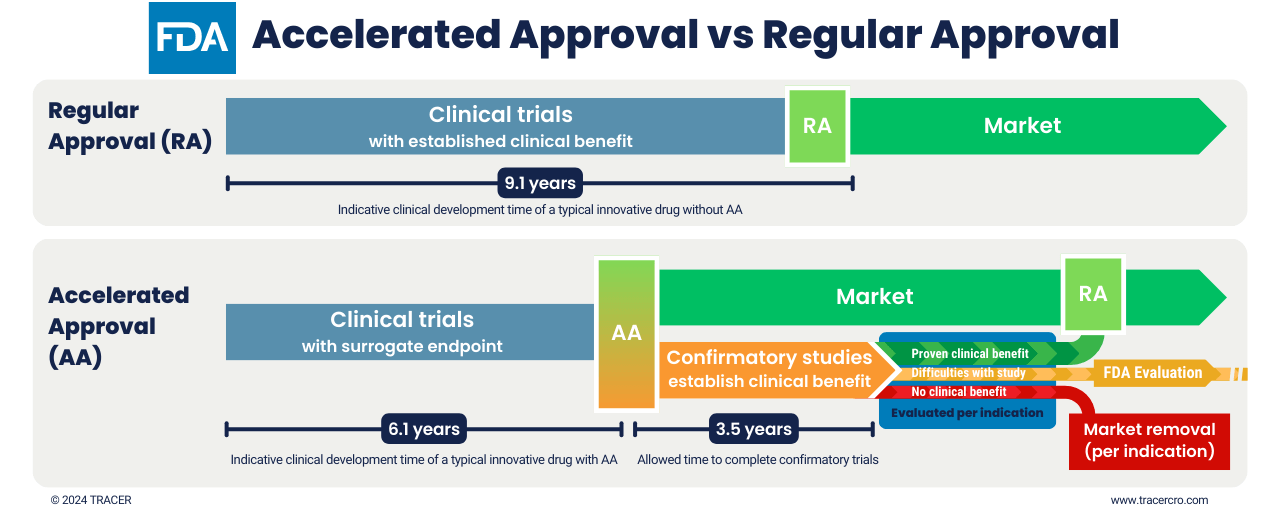
FDA accelerated approval process vs regulator approval
Accelerated Approval gets a drug about 3 years earlier on the market. The permission for market entry is based on surrogate endpoints. Multiple indications may be included. While already on the market, a confirmatory clinical trial must be conducted. The FDA sets the timeline, milestones, and other study requirements. In this clinical trial, the surrogate endpoints should translate to clinical benefits. Evaluation is done per indication for which the drug received accelerated approval. If clinical benefits are established only for certain indications, the drug can obtain regular market approval for those indications.
Failure of confirmatory studies does not always lead to market removal
Absent or insufficient results of a confirmatory study can be a reason to withdraw the drugs from the market or remove indications from the label. However, the FDA will discuss this with the sponsor. The FDA can also revise milestones, explore alternative trial designs, or accept delays in study conduct. Decisions are made on a case-by-case basis. It largely depends on whether the conditions for accelerated approval still exist and what kind of problems there are with a particular study. Recent amendments to the AA program provide the FDA with more control regarding confirmatory studies and market removal.
Examples of failure in confirmatory clinical trial leading to extension
Between 2012 and 2021 there were several cases with a confirmatory trial FDA acceptance for delay. Three of the accepted delays were due to COVID-19; presenting studies with difficulties regarding study start-up and inclusion.
- In two cases the confirmatory trial phase was delayed due to delays in other reliant studies.
- Four therapeutic confirmatory trials were extended so more data could be obtained.
- In three other cases, there were delays due to protocol reconciliation.
Accelerated oncology clinical trials
Oncology focused therapies are dominant in the accelerated program, accounting for 80% of approvals since 2006. Surrogate endpoints in oncology often include tumor shrinkage. However, the list of Accelerated Approval FDA surrogate endpoints also includes:
- major hematologic response;
- major hematologic response and cytogenic response;
- durable complete remission rate;
- durable complete response rate;
- durable objective overall response rate (ORR);
- metastasis-free survival;
- progression-free survival (PFS);
- disease-free survival (DFS);
- event-free survival (EFS);
Endpoints available for your drug depend on the disease, patient population, and drug Mechanism Of Action (MOA). Usage of these or other endpoints should always be discussed with the relevant regulatory bodies.
FDA Accelerated Approval history
FDA accelerated approval vs full approval
The major differences between accelerated approval and full approval are listed below:
|
Accelerated |
Regular |
|
|
Endpoints |
Surrogate |
Clinical benefit |
|
Indication |
Serious or life-threatening diseases |
All |
|
General time between IND and market |
Shorter |
Longer |
|
Postmarketing Commitments |
Confirmatory studies |
Post market surveillance |
|
FDA label requirements |
Accelerated Approval indication |
Regular |
Discuss expedited drug development with TRACER
TRACER CRO works with life sciences and biotech companies to bring their technologies faster to market. We do this with two main strategies. First of all, we use regulatory pathways for expedited development. Accelerated approval in the US or Conditional Marketing Authorization in the EU are only a few of the options. Second, as a contract research organisation, we provide innovative solutions. These include exploratory studies in patients (Phase 0), Phase 1b and 2a, and imaging trials. We are happy to discuss the possibilities of accelerated approval and surrogate endpoints available to you. Contact us to request more information or schedule a meeting.
Frequently asked questions
We are happy to answer some frequently asked questions about accelerated FDA approval. For more information and answers to your questions, please contact us. Accelerated Approval is a program by the FDA. The European Medicines Agency (EMA) equivalent for this conditional approval pathway is EMA Conditional Marketing Authorization (CMA). Similar options are available in the EU for other FDA fast-track programs. The FDA accelerated approval program is a unique program that provides drugs early access to the market without proven clinical benefit in the standard phases of clinical trials. Moreover, the FDA grants accelerated approval based on surrogate endpoints. These surrogate endpoints predict clinical benefit, but an after-market-entry mandatory confirmatory study should demonstrate clinical benefit. Upon success, regular approval will be granted. However, if clinical benefit is not sufficiently demonstrated, or the confirmatory study is for other reasons unsuccessful, there is a procedure for market removal. There are 4 FDA expedited programs, where the FDA accelerated approval program is the only alternative pathway. The other three are merely guidance and accelerated review. Depending on meeting the criteria, a drug may receive FDA expedited program guidance, accelerated review, and/or Accelerated Approval. Read more about the Expedited programs on the FDA website, or discuss possibilities with TRACER. Accelerated approval guidance is only available if program criteria are met and surrogate endpoints are available with sufficient evidence to be used as a predictor of clinical benefit. The FDA decides this on a case-by-case basis. An Investigational New Drug (IND) designation needs to be granted by the FDA before clinical trials can take place. Obtaining IND approval can take up to 30 days and is based on non-clinical (safety) data. Before market-entry of a drug, the FDA will decide on a New Drug Application (NDA) or Biologic License Application (BLA). For this, the preliminary review phase takes up to 60 days. After a positive preliminary review, standard FDA approval can take up to 10 months. FDA Priority Review reduces this time to 6 months. FDA Priority Review alone does not change clinical trial requirements like Accelerated Approval. Accelerated Approval can be combined with other fast-track programs. You can see if a drug is on the list of accelerated approval drugs by AA or AAP indication.
Does EMA offer Accelerated Approval?
What is FDA accelerated approval program?
What are the 4 FDA expedited programs?
Is FDA accelerated approval guidance available?
How fast is FDA approval?
How many drugs have been approved by accelerated approval?
There is an FDA accelerated approval list, that can be found here:
https://www.fda.gov/drugs/nda-and-bla-approvals/accelerated-approvals.
Between 1992 and 2021, there have been 278 accelerated approvals. From 1992 to December 2021, only 32 were withdrawn and 107 are still in the phase of confirmatory studies. A study (Benedict et al., 2024) shows that between 2006 and 2021, 911.000 patients received AA oncology drugs. This resulted in an estimated 263,000 additional life years gained.
Conclusion
Accelerated Approval is a fast-track FDA program bringing drugs faster to patients who are often in desperate need. The use of surrogate endpoints and early-market access combined with confirmatory studies has proven to speed up the drug development process. Amendments to the program have provided the FDA with more control. Furthermore, the high prices of AA drugs combined with unproven clinical benefits, can negatively influence the public opinion of these drugs. Given the delay in confirmatory studies and the lack of AA indication on labels, a higher level of FDA oversight may be deemed necessary.
Abbreviations
| FDA | U.S. Food and Drug Administration |
| AA | Accelerated Approval |
| AAP | Accelerated Approval Program |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| HRQoL | Health-Related Quality of Life |
| EMA | European Medicines Agency |
| RA | Regular Approval |
| ORR | Overall Response Rate |
| PFS | Progression-Free Survival |
| DFS | Disease-Free Survival |
| EFS | Event-Free Survival |
| MOA | Mechanism Of Action |
| FDAMA | Food and Drug Administration Modernization Act |
| FDASIA | Food and Drug Administration Safety and Innovation Act |
| IND | Investigational New Drug |
| CRO | Contract Research Organisation |
| CMA | Conditional Marketing Authorization |
| NDA | New Drug Application |
| BLA | Biologic License Application |
Citations
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9869766/
Mozersky J, Roberts JS, Rumbaugh M, Chhatwal J, Wijsman E, Galasko D, Blacker D; AGREED. Spillover: The Approval of New Medications for Alzheimer’s Disease Dementia Will Impact Biomarker Disclosure Among Asymptomatic Research Participants. J Alzheimers Dis. 2022;90(3):1035-1043. doi: 10.3233/JAD-220113. PMID: 35404285; PMCID: PMC9794032.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9794032/
Benedict, Á., Szabó, G., Marczell, K., Doherty, B., & Martin, S. (2024). Life Years Gained From the FDA Accelerated Approval Program in Oncology: A Portfolio Model. Journal of the National Comprehensive Cancer Network (published online ahead of print 2024). Retrieved May 15, 2024, from https://doi.org/10.6004/jnccn.2024.7010
Ballreich J, Socal M, Bennett CL, Xuan A, Trujillo A, Anderson G. Accelerated approval drug labels often lack information for clinical decision-making. Pharmacotherapy. 2023; 43: 300-304. doi:10.1002/phar.2789
https://accpjournals.onlinelibrary.wiley.com/doi/10.1002/phar.2789