Early phase clinical trials
Early phase clinical trials are first in-human trials, meaning it is the first time the investigated drug will be administered to humans. Early phase clinical research knows two distinct strategies. Traditionally early clinical development starts with Phase I. Due to a high failure rate of 66.4% in Phase I (Wong et al., 2019), the FDA and EMA encourage Phase 0 studies before starting Phase 1. Not only can this exploratory clinical trial increase the chance of success, but it can also reduce cost and time in drug development. TRACER specializes in early phase clinical trials in patients.
Let’s bring your drug faster from bench to bedside! Discuss your early-phase possibilities with us. TRACER’s Phase 0 exploratory trials deliver in-patient data approximately 1-2 years earlier than the conventional method. Contact us today.
Your first early phase clinical trial
Early phase clinical trials are very exciting for drug developers, especially the first in human study. Why? Well, this translational research aims to reproduce and validate the findings from laboratory studies, such as animal models and cell lines, in humans. For this pivotal clinical trial, drug developers have the choice of two different clinical trial approaches. First conduct Phase 0, or start with Phase 1 instead.
TRACER CRO
Early phase clinical trials execute the translation from preclinical to clinical. Our services as an early phase CRO cover the spectrum of preclinical and early clinical (up to Phase 2). By utilizing the possibilities of Phase 0 and imaging, we can replace part of the preclinical work with an early in-human study. We are a full service CRO, meaning we take care of everything. From obtaining regulatory approval, patient recruitment, and study design to data analysis and study writing.
Faster from bench to bedside: TRACER’s mission
TRACER has one goal: to make scientific discoveries faster available to patients. Every day we remind ourselves why we do what we do: make a difference in the lives of patients and their loved ones. To fulfill our mission, we help drug developers bring their drugs to market as fast as possible. We reduce their chance of failure with smart, often adaptive, study designs. We use the new possibilities in early clinical trials to study the behavior of a drug in-patient. Allowing our clients to use in-human screening of compounds and obtain PK/BD data through imaging.
When should I contact TRACER?
You can contact us at any time. The sooner we can initiate early phase clinical trials, the more benefits you get. We can simultaneously prepare your Phase 1 clinical trial while conducting the Phase 0 exploratory trial. Also, part of your preclinical work can be replaced with early clinical development. A good example of this, is in-human selection of lead compound. In any development stage, you can request a meeting to discuss your clinical translation. From this meeting, you will get a complete picture of your drug development through the eyes of a CRO.
Late-preclinical and early phase clinical trials
Our services range from late-preclinical to early phase clinical trials. As stated earlier, we are a full service CRO, meaning we can run your project entirely.
Drugs for patients, not for healthy volunteers
We are making drugs for patients, not for healthy volunteers. So why, before knowing how the drug behaves in patients, spend time and resources on clinical trials with healthy volunteers? With the knowledge that around 33% of Phase 1 studies fail, and around 50% of Phase 2 studies fail, why expose participants to this unnecessary risk? Phase 0 can identify a lead compound and the patient population for which it is most promising. The early go/no-go decision after Phase 0 can prevent unnecessary exposure for participants.
Phase 0
Phase 0 exploratory clinical trial
Phase 0 is a first in patient study that is also referred to as exploratory trial, early clinical, early Phase 1, or microdosing study. This study comes before Phase 1 and is a relatively small and quick clinical trial. The study provides PK/BD data based on a microdose of the compound. It is a translational clinical trial in patients. This means that the obtained data can be used in the safety assessment of Phase I and indication/patient selection in Phase I or Phase II. Phase 0 is not only quick in study conduct, but due to less strict regulatory requirements, study approval is also fast.
Phase 0 is available for all types of compounds
Phase 0 is available for all types of compounds.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Types of Phase 0 early stage clinical trials
Within the Phase 0 framework, there are several different possibilities for your early stage clinical trial. Phase 0 is providing drug developers with a truly additional route in translational drug development.
In essence, the two distinct objectives are:
- Phase 0 compound screening for lead candidate selection
- Phase 0 Proof of Concept for early in-patient PK/BD, MOA, ADME data
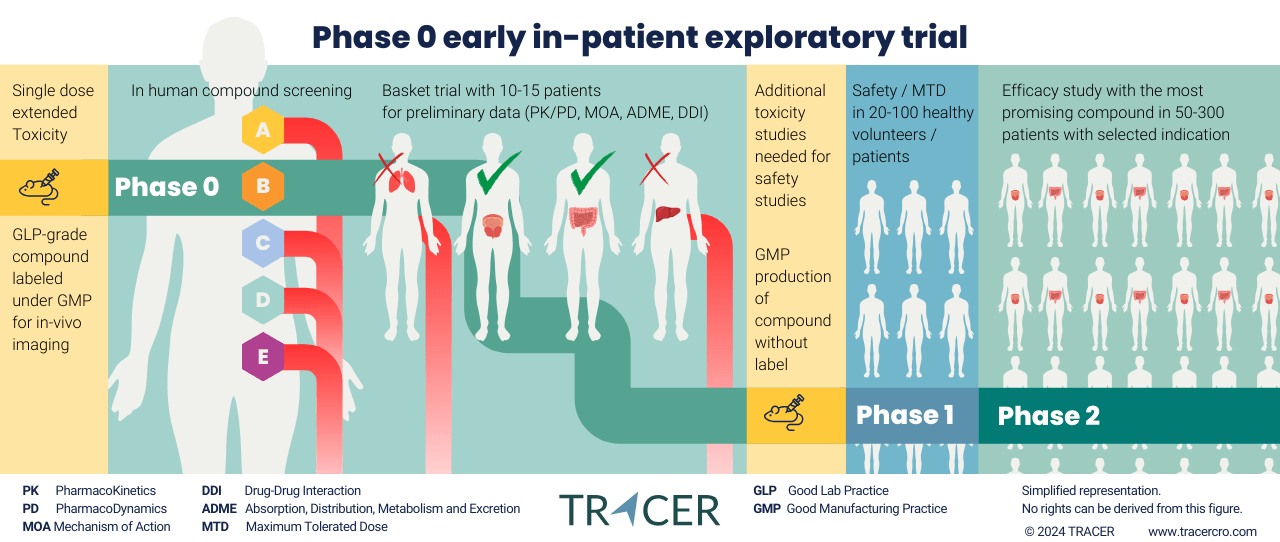
Phase 0 screening for lead candidate selection (cassette microdosing)
The drug discovery phase leaves drug developers often with multiple compounds or variations of one compound. A Phase 0 screening study (cassette microdosing) can provide conclusive evidence to decide on the lead candidate when preclinical data is conflicting or insufficient. The Phase 0 clinical trial is an exploratory trial, meaningful endpoints can be identified that could lead to a decision. E.g. bioavailability, half-life, biodistribution, target binding, off-target binding, and accumulation. Moreover, Phase 0 screening may replace part of preclinical screening.
Objectives of studies in early phase clinical research
Early phase clinical research provides data on safety and dosing that can be used in Phase II. To optimize your Phase II study design, use the early phase clinical trial to study:
- Pharmacokinetics (PK)
- Pharmacodynamics (PD)
- Target binding
- Receptor occupancy
- Postreceptor modulation
- Mechanism of Action (MOA)
- Absorption, Distribution, Metabolism and Excretion (ADME)
- Half-life
- Bioavailability profile, such as concentration – time curve
- Effect on biological elements, such as enzymes, transporters and genomics
- Drug-Drug Interactions (DDI)
- Variability
Reasons to conduct a Phase 0 study
- Obtain preliminary data
- Lead candidate selection
- Study metabolites
- Study human diseases without adequate in-vivo/in-vitro/in-silico (animal) models
- Use microdosing for drugs with toxicity/safety concerns
- Study in vulnerable patients, paediatric patient study
- Test enteric drugs by parenteral administration, allowing absolute bioavailability
Phase 0 FAQ
What are Phase 0 ICH guidelines?
ICH Guideline M3 (R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals describes 5 approaches to exploratory clinical trials.
Review the 5 approaches outlined in the ICH guideline M3(R2).
What is the window of opportunity Phase 0 in neuro-oncology
The window of opportunity trial in neuro-oncology is very different from other Phase 0 trials. Patients with for instance glioblastoma can receive a full therapeutic dose about one week before surgery. After tumor resection, analysis can show if the drug successfully penetrated the blood-brain-barrier and if there was target uptake/effect. The reason for this study design, is the large amount of drug that show positive results in animal models, but fail in-human. If the Phase 0 window-of-opportunity trial is successful, the patient is able to enroll in Phase II and continue the novel treatment (den Bent, 2020).
Phase 1
Phase 1 studies in early phase clinical trials
The classical Phase 1 study has a straightforward study goal; do a safety and dosing assessment to define the therapeutic window that can be used for the Phase II trial. Common studies conducted within the scope of Phase 1, are:
- Dose Escalation Studies
- SAD study (Single Ascending Dose)
- MAD study (Multiple Ascending Dose)
- Food effect study
- QT study / TQT study (Thorough QT) / QTc study (“c” stands for correlated)
- Bioavailability study
- Human Mass Balance study
- ADME study
- Quantitative Tissue Distribution study (Quantitative Whole-Body Autoradiography, or for short, QWBA study)
- PK study
- PD study
By optimizing the study design in Phase 1, valuable data for your Phase II trial can be obtained. The division in Phase Ia and Ib capitalizes on this.
Phase 1 FAQ
What is a Phase 1 clinical trial?
Phase 1 is the first in-human tolerability study to assess safety, side effects, dosing, and timing of administration. Phase 1 is generally conducted on healthy volunteers, although early phase oncology clinical trials are directly in patients. Phase 1 does not assess efficacy but can provide information on drug distribution and accumulation.
What is the phase 1 success rate?
Study shows that the probability of success in Phase I is 66,4%. This means, approximately 1 in 3 investigational drugs don’t make it from Phase I to Phase II. There are several reasons for failure. Partially this is due to unacceptable toxicity levels. Learning more about Pharmacokinetics (PK) and Biodistribution (BD) from Phase 0, before Phase 1, can be beneficial to overcome unpredicted symptoms or shortcomings in study design.
What are the stages of early phase clinical research? (updated)
What are the early phases of clinical trials? Only Phase I and 2 were traditionally defined as the early phases of drug development. Two decades ago, Phase 0 was introduced by the regulatory authorities. Since then, Phase 0, I, and II are considered early clinical. In recent years early phase clinical research has been further divided into Phase 0, Ia, Ib, IIa, and IIb.
What is the difference in Phase 1a vs Phase 1b?
Phase 1 can be divided into Phase 1a and Phase 1b. Phase 1a is often a SAD study with healthy volunteers. Phase 1b is a MAD study, also with healthy volunteers. After establishing initial safety in Phase 1a, Phase 1b allows drug developers to expand dose-finding. Phase 1b can also include patients. In certain cases, the complete Phase 1 study is conducted in-patient, and trials with healthy volunteers are skipped.
When are Phase 1 studies in patients instead of healthy volunteers?
For most INDs Phase 1 safety and dosing studies are conducted in healthy volunteers. There are guidelines on when conducting Phase 1 in patients is acceptable. It depends on the benefit/risk balance.
- The risk considered acceptable for healthy volunteers is lower than for patients with a serious or life-threatening disease.
- The risk of causing illness in healthy volunteers should be low.
For instance, due to the unpredictable and often toxic nature of the compound anti-cancer clinical trials differ from other Phase 1 trials. They are commonly conducted in patients. In addition to early phase oncology clinical trials, other trials might also be directly in-patient.
Are Phase 1 patient trials possible for other, non-cancer, drugs?
Although it should be evaluated for each individual case, Phase 1 can be directly in-patient when any of the following characteristics are present:
- unpredictable toxicity;
- cytotoxicity;
- cytostaticity;
- immunosuppressant;
- biological products, such as antibodies, vaccines, gene products, cell therapy, growth factors;
- anti-viral / infection;
- small molecule drug.
Considerations for a Phase 1 first in-patient study
- The disease can influence PK/BD/tolerability.
- There can be a potential Drug-Drug-Interaction (DDI study).
- Slow inclusion when there is a low quantity of patients able and willing to participate.
- Ensure data quality and representation.
Phase 2
Phase 2 trials
The scope of Phase 2 clinical trials is evaluating efficacy in a large group of patients. The sample size is typically 50-300 participants. When Phase 1 has been conducted solely in healthy volunteers, phase 2 is the first in-patient clinical trial. When Phase 2 clinical trials are divided into Phase 2a and 2b, the first can be seen as a pilot phase for dose finding and continuation of safety assessment in-patient. Phase 2b is focused on efficacy.
Which phase for your early clinical development?
Phase 0 or Phase I can both be chosen as the first in human study, but they are very different from each other. This article discusses the options for early clinical development and translational research. However, to learn which option is best for your specific compound, contact TRACER.
Early phase clinical trials: Phase I vs Phase 0
What is the difference between Phase 1 and Phase 0? When you’re new to early phase clinical trials it’s good to make the comparison. To decide on your early phase trials, we compare Phase I vs Phase 0. As an early phase CRO, we can assist you with both phases, as well as with subsequent phases. As a take-away, Phase 0 is often the best starting point. This should be no surprise; Phase 0 was introduced for a very good reason. We’ll get to that later. First, let’s compare Phase 0 and Phase 1.
What is the difference between Phase 0 and Phase 1?
This table will point out the differences between Phase 0 and Phase 1 based on the standard application. Individual studies may differ from the standard.
| Phase 0 | Phase 1 | |
| Main study objective | Proof of Concept/Lead compound selection | Safety / Dosing |
| Sample size | 10-15 | 20-100 |
| Participants | Patients | Healthy volunteers (only in certain cases patients) |
| GLP/GMP material | GLP | GMP |
| Timeline | 14-18 months | 1-2 years |
| Tox | Single dose extended toxicity study | Full tox study |
| Dose | Subtherapeutic microdose, or IntraTarget Microdose (ITM) at local and temporary therapeutic levels | Therapeutic window: therapeutic dose up until Maximum Tolerated Dose (MTD) |
| Regulations | More lenient | Strenuous |
| Clinical Pharmacology Units (CPU) needed | More often not | Often yes |
| Costs | Lower | Higher |
| Participant recruitment | Via medical facilities | Recruitment of healthy volunteers |
What is a microdose?
A microdose is, depending on what is lowest:
- <100 μg or for proteins, the maximum amount is <30 nmole;
- 1/100 of No Observed Adverse Effect Level (NOAEL);
- 100th of the Pharmacologically Active Dose (PAD);
- For ITM microdose scaled on milligrams per kilogram body mass, the physical target should be <1/100th of total body mass;
- For oral administration scaled, on milligrams per square meter.
NOAEL for 14 days Area Under the Curve (AUC) in non-rodent models or ½ NOAEL AUC in rodent models, whichever is lowest.
Early phase clinical trials and their scope
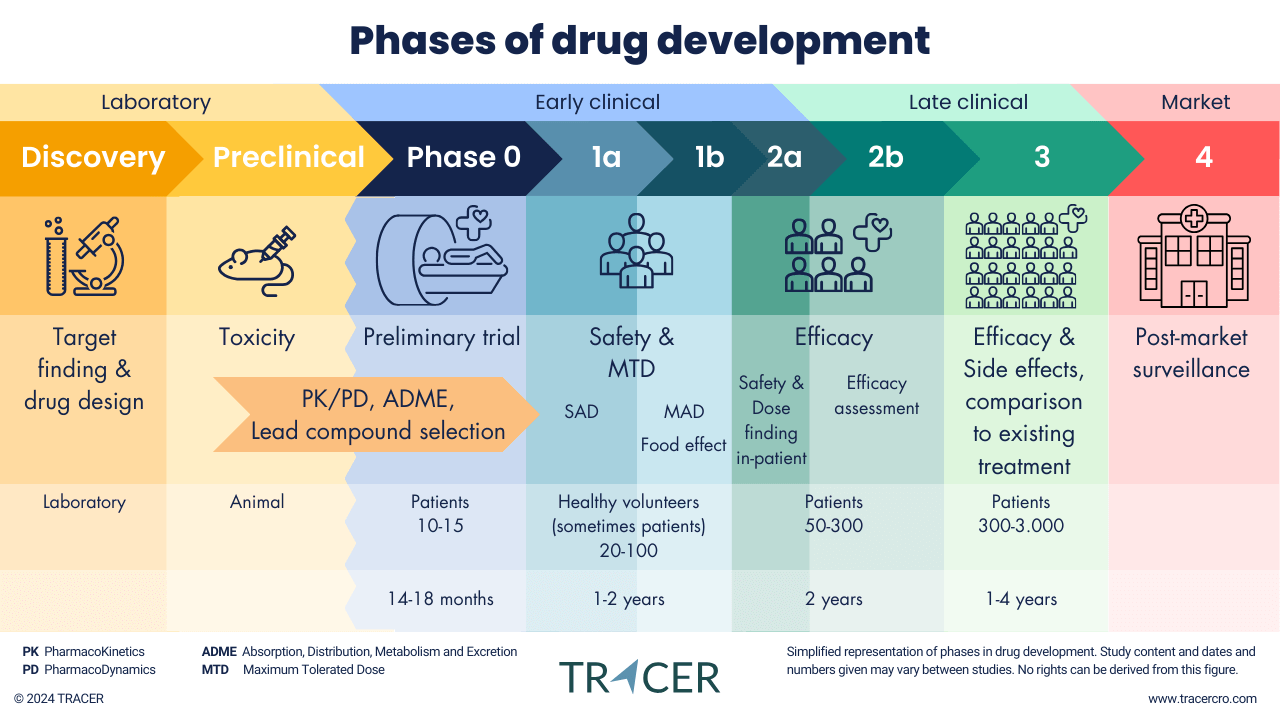
In the table below we describe the early development clinical trials in steps.
| Phase | Goal | Dose | Participants | Timeframe in months |
|---|---|---|---|---|
| Phase 0 | Study PK/BD | Microdose | 10-15 Patients | 14-18 |
| Phase I | Assess preferred method of administration, safety, tolerability, timing, and side effects | Dose escalation (can be studied in combination with (T)QT and Food effect) | 20-100 Healthy volunteers (or only in certain cases, patients) | 12-24 |
| Phase Ia | Assess PK safety levels | Single ascending dose (SAD) | 20-100 | – |
| Phase Ib | PK/PD study and Food effect study | Multiple ascending dose (MAD) | 10-20 | – |
| Phase II | Study biological activity | Therapeutic dose | 50-300 patients | 12-24 |
| Phase IIa | Optimal dose finding and safety assessment in patients | Therapeutic dose | 20-100 | – |
| Phase IIb | Efficacy assessment | Therapeutic dose | 200-300 | – |
The provided information is according to the general study conduct, individual studies can differ.
Strategies to get the most out of your early phase trial
The early development clinical trials are a great chance to obtain valuable data for later trials. However, because Phase II is the more important trial, we want to keep the early clinical trial trajectory, Phase 0-1, as short as possible.
- Choose a trial design that minimizes the risk of delay.
- Phase 0 can be used to screen multiple compounds in-human.
- A basket trial can provide data on multiple indications.
- Use a clinical study design to anticipate on following studies.
- Use Phase 0 / 1b / 2a to gather in-patient data before a full-size Phase 2 trial.
Phase 0, Ib, and IIa: proof of concept studies
Phase 0, Ib and IIa are all Proof of Concept studies. They aim to provide data to increase the success rate in subsequent clinical trials. Phase 0 is the best translational clinical trial since it can provide early clinical PK/BD data. This data can be used to design the Phase I study smarter. If Phase I is already chosen as first in human trial, Phase Ib can be added to gain insight into PK/BD. Again, this data will be used to design the next study, in this case Phase 2. Phase 2b functions as a dosing study in patients to assess safety, tolerability, and efficacy in patients.
Phases of clinical trials vs studies
Good to know: the phases of clinical trials are merely a guideline. Each phase represents different kinds of studies that drug developers should conduct. There is a certain chronology, hence the phases, but there is room for creativity. A study design may already anticipate the subsequent phase. At TRACER we use this to obtain data needed for the design of the next study from the previous one. As an early phase CRO, we often conduct all early phase clinical studies for our clients.
Accelerate your early phase drug development
Time lost in preclinical and clinical will reduce the time left on your patent once your drug enters the market. Nowadays there are many possibilities to accelerate your early phase drug development. You only need to know what options are available to you. We are happy to guide you along the way.
Frequently asked questions
Below we answer some of the frequently asked questions regarding early phase clinical trials. If you have another question, feel free to contact us.
What is an early phase 1 study?
Early Phase 1 study is another name for Phase 0. The same applies to exploratory trial, early clinical, and microdosing study. Meaning, a study conducted before the commonly known Phase 1 study in healthy volunteers (or patients). Most important to understand, is that there are more options available in drug development than the classical Phase 1 study as first in-human trial (Berman et al., 2017).
What is causing problems with participant inclusion for early phase clinical trials?
Inclusion is one of the reasons clinical trials tend to fail. Early phase clinical trials have a high risk of inclusion problems. There are several reasons for this.
- Healthy volunteers in Phase 1 studies, and patients receiving a non-therapeutic dose in Phase 0 cannot expect health benefits.
- Phase I studies are evaluating for the first time safety for humans and therefore the perceived risk might be considered too high.
- Drug developers moving a compound into the clinic for the first time, are often tempted to set the inclusion criteria too strict, resulting in few eligible subjects and a slow accrual rate.
What is the difference between early and late phase clinical trials?
Early phase clinical trials are still determining safety, dosing, and efficacy. Late phase clinical trials compare the investigational drug to existing treatment and monitor side effects.
| Early phase | Late phase | |||
| Phase 0 | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
Early phase meaning the investigational new drug is still not proven effective and safe. Late phase means the drug is considered effective and safe, but there is not yet enough data available. Therefore, late phase trials are conducted with many participants and are therefore more expensive.
What are the 3 types of clinical trials?
Technically there are more than 3 types of clinical trials. Depending on the criteria, you can identify 4 phases of clinical trials and 1 phase of Post-Market Surveillance (PMS). Depending on the criteria, clinical trials can be divided in:
- Safety assessment in Phase I
- Efficacy assessment in Phase 2
- Comparison to standard treatment in Phase 3
Or, with 4 Phases:
- Preliminary PK/BD Proof of Concept study / Compound screening
- Safety and dosing
- Efficacy
- Comparison
In this case, the PMS study becomes Phase 5 in drug development.
In theory, PMS never ends since it is based on real-life evidence such as adverse event reporting.
What is Phase 1 vs Phase 2 vs Phase 3 clinical trials?
The following table explains Phase 1 vs Phase 2 vs Phase 3 in clinical trials in general terms, without nuancing.
| Phase 1 | Phase 2 | Phase 3 | |
| Study objective | Safety and dosing | Efficacy in patients | Comparison to existing treatment |
| Type of participants | Healthy volunteers | Patients | Patients |
| Qty. of participants | 20-100 | 50-300 | 300-3.000 |
| Dosing | Ascending doses | Therapeutic dose | Therapeutic dose |
| Success rate (Wong et al., 2019) | 66.4% | 48.6% | 59% |
| Time in years | 1-2 | 2 years | 1-4 |
| Estimated costs in $ in millions
(Sertkaya et al., 2016) |
1.4-6.6 | 7-19.6 | 11.5 – 52.9 |
Compare this to a Phase 0 study, which is conducted directly in 10-15 patients, taking around 14-18 months and costing between $ 400K-1.2M.
Abbreviations in this article
|
ADME |
Absorption Distribution Metabolism and Excretion |
|
AUC |
Area Under the Curve |
|
BD |
BioDistribution |
|
CPU |
Clinical Pharmacology Unit |
|
CRO |
Contract Research Organisation |
|
DDI |
Drug-Drug Interaction |
|
EMA |
European Medicines Agency |
|
FDA |
Food and Drug Administration (USA) |
|
GLP |
Good Laboratory Practice |
|
GMP |
Good Manufacturing Practice |
|
ICH |
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
|
ITM |
IntraTarget Microdose |
|
MAD |
Multiple Ascending Dose |
|
MOA |
Mechanism of Action |
|
MTD |
Maximum Tolerated Dose |
|
nmole |
nanomole |
|
NOAEL |
No Observed Adverse Effect Level |
|
PAD |
Pharmacologically Active Dose |
|
PD |
PharmacoDynamics |
|
PK |
PharmacoKinetics |
|
PMS |
Post-Market Surveillance |
|
QT |
Interval from the beginning of the QRS complex to the end of the T wave |
|
QTc |
QT corrected |
|
QWBA |
Quantitative Whole-Body Autoradiography |
|
RFI |
Request For Information |
|
SAD |
Single Ascending Dose |
|
TQT |
thorough QT |
|
μg |
microgram |
Berman, A. Y., Motechin, R. A., Wiesenfeld, M. Y., & Holz, M. K. (2017). The therapeutic potential of resveratrol: a review of clinical trials. Npj Precision Oncology 2017 1:1, 1(1), 1–9. https://doi.org/10.1038/s41698-017-0038-6
den Bent, van. (2020). Phase 0 and Window of Opportunity Clinical Trial Design in Neuro-Oncology: A RANO Review. Neuro-Oncology, 22(11), 1568–1579. https://doi.org/10.1093/neuonc/noaa149
Sertkaya, A., Wong, H. H., Jessup, A., & Beleche, T. (2016). Key cost drivers of pharmaceutical clinical trials in the United States. Clinical Trials, 13(2), 117–126. https://doi.org/10.1177/1740774515625964/SUPPL_FILE/625964_SUPP_MATERIAL.PDF
Wong, C. H., Siah, K. W., & Lo, A. W. (2019). Estimation of clinical trial success rates and related parameters. Biostatistics, 20(2), 273–286. https://doi.org/10.1093/BIOSTATISTICS/KXX069
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, clinical images, or copyrighted terms wherein.