Nuclear medicine and molecular imaging allow drug developers to study the behavior and distribution of their compound in-human at an early stage. Namely, medical imaging in clinical trials visualizes on-target and off-target binding data. PET, SPECT, CT, and MRI clinical trials provide a solution to the increasing pressure to develop novel drugs at a lower cost and fast pace. TRACER specializes in nuclear imaging clinical trials. Continue reading to learn how our imaging solutions change the drug development industry. Contact us to discuss how this can help you as a drug developer.
Contact us
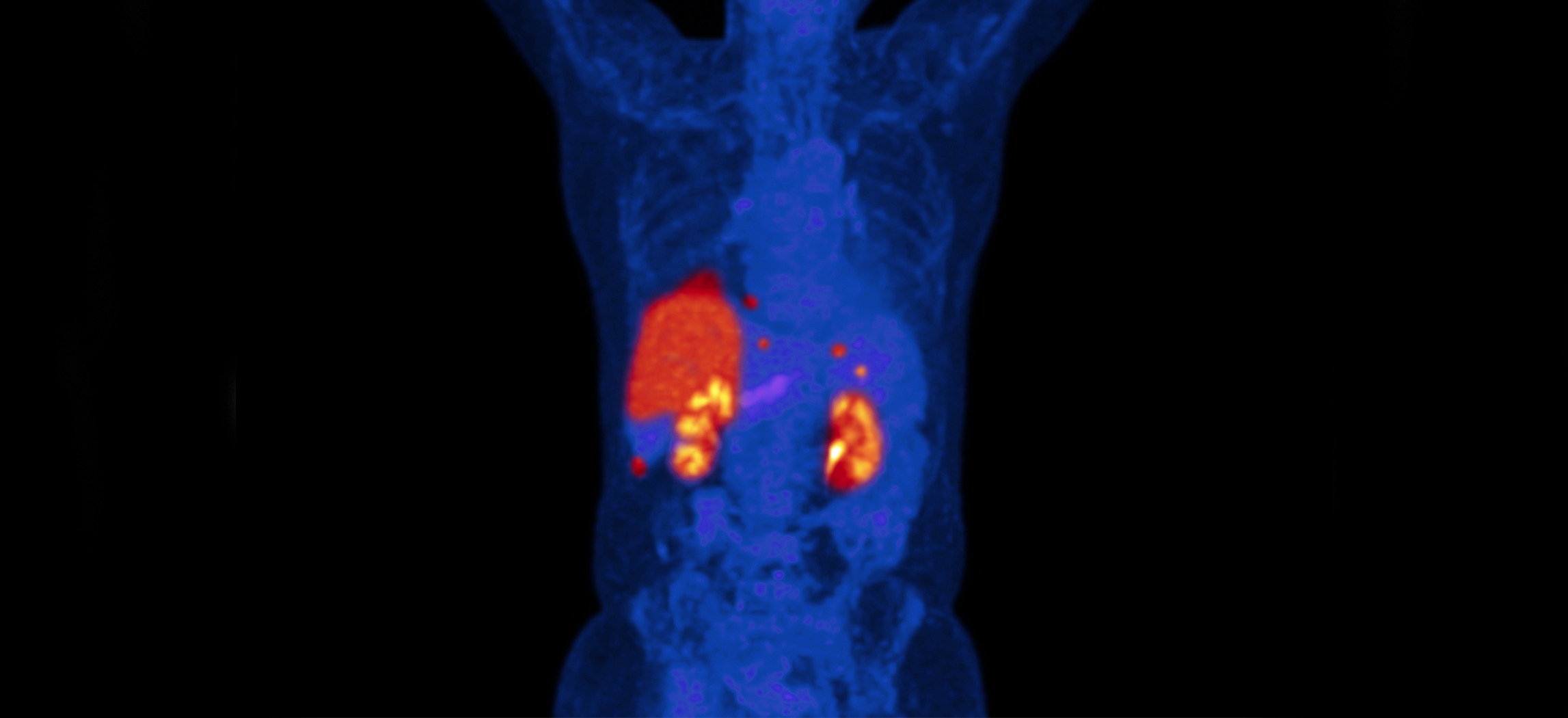
Medical imaging in clinical trials is changing the industry
With SPECT or PET imaging, early in-human data on the dynamics of a drug can be obtained. This data allows for enhanced decision-making before moving the compound to the next development phase where costs are larger and valuable time is lost. Imaging can benefit all phases of clinical trials, but Phase 0 clinical trials are the real game-changer. Adding imaging to early phase clinical trials, such as Phase 0, means you know if your compound reaches and binds to the target tissue and where side effects can be expected. A Phase 0 study can be conducted in approximately 12-18 months. No other phase of clinical trials is that fast and provides that level of accuracy.
What does Nuclear Molecular NM mean?
Did you know: the abbreviation for Nuclear Medicine is NM. Of course, NM can mean more than Nuclear Medicine, but if you hear NM imaging, like an NM bone scan, you know that radioactive pharmaceuticals are used.
Radiopharmaceuticals, terminology in nuclear molecular imaging
Radiopharmaceuticals can refer to radiolabeled pharmaceuticals for imaging or radioactive pharmaceuticals for therapy. The latter includes drugs used for radiation therapy, where the emitted radiation is intended to damage cells, such as cancerous cells. Radiolabeled pharmaceuticals for imaging do not intend to harm cells but to emit a small amount of radiation to generate images during PET and SPECT scans. This method has proven benefits for developing new drugs.
Radiopharmaceuticals in nuclear medicine and clinical trials
With a few exceptions, any type of antibody, molecule, protein, or other compound can be nuclear labeled. This means that an investigational new drug can be labeled with a radioisotope for imaging during the clinical trial. The drug is only labeled for imaging and will be used without the label for future therapeutic use. It is, of course, also possible to conduct a clinical trial for nuclear medicine that will be used in radiation therapy.
TRACER mission
The main objective of TRACER is to get new drugs to patients faster. Providing our sponsors with enough time left on their patent and more market time while reducing R&D time and costs in drug development in general.
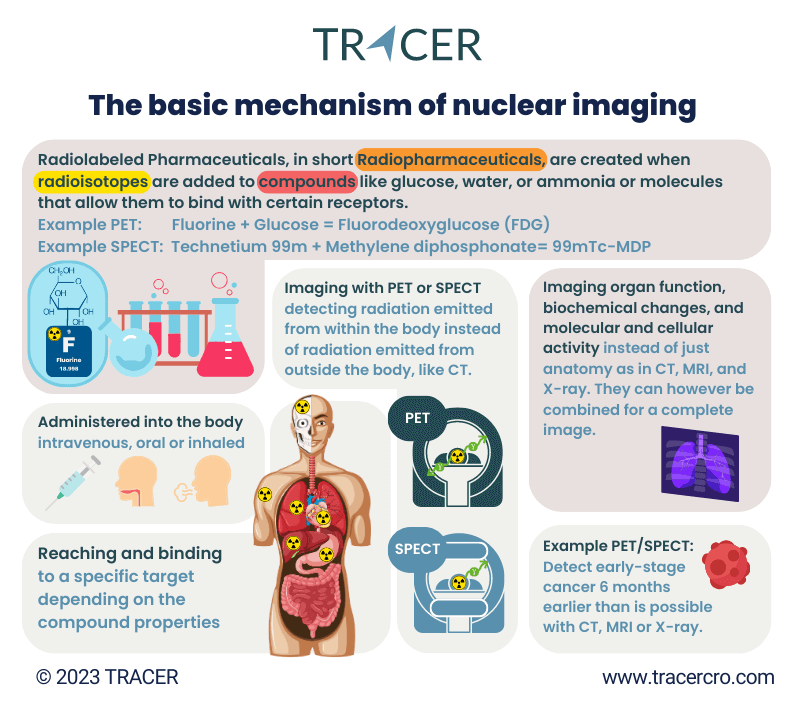
What is meant by nuclear imaging?
There are multiple methods of molecular imaging for clinical trials. The most common methods are optical fluorescence imaging and nuclear imaging. Read more about fluorescence imaging.
What is meant by nuclear imaging? Nuclear imaging uses radioactive pharmaceuticals and scanners to visualize processes in the body. A radioactive pharmaceutical can simply be a radioisotope that accumulates in the target of interest. For example, the use of a radioactive substance, radioactive isotopes of iodine, for the detection and treatment of thyroid cancer. Or a radioactive tracer for PET or SPECT that is coupled to a novel drug to add to the clinical trial imaging.
Clinical trials images
There is a reason that radioactive substances are used to add to clinical trials imaging. Since gamma rays have high penetration characteristics in tissue, nuclear imaging methods are suited for the detection of lesions seated deep in the body. In contrast with fluorescence imaging and ultrasound techniques, which have a limited penetration depth. When comparing nuclear vs optical imaging for your clinical trial, penetration depth can be a decisive factor.
Targeting agents
Another type of radiopharmaceuticals is the use of targeting agents (small molecules, peptides, antibodies, etc.) conjugated with a radioisotope. By adding a radioisotope nuclear imaging can be used for diagnostics, treatment, and drug development. You need to understand that there is not just one method of nuclear imaging, there are several methods and many different sorts of radioactive medicines. Each of them has specific binding and targeting attributes.
Start with nuclear labeling as early as possible in your research
Traditional drug development follows several, fixed phases. In recent years, there have been many discussions about this way of working from all industry stakeholders, including from commercial organizations and regulators.
As many as 90% of investigational new drugs fail in clinical trials, especially in the later and more expensive phases. Introducing molecular imaging as early as possible into clinical research can make extensive preclinical studies on animal models redundant. Namely, molecular imaging for drug development using a microdose can be done directly into the target population. Meaning drug developers can already go into clinical before Phase 1 and skip expensive large animal models.
Cellular imaging in drug discovery
Labeling your compound with a radionuclide and testing this nuclear medicine in-patient as early as possible, can speed up your drug development process. Molecular imaging of radioactive material in-patients provides reliable pharmacokinetics/biodistribution (PK/BD) data, while ex-vivo or in-vitro may be biased. Cellular imaging in drug discovery is possible with in-vivo imaging such as using PET or SPECT. PET and SPECT can be used to make a whole-body nuclear scan. Ex-vivo or in-vitro microscopy can be a solution in preclinical drug discovery.
How to set up your preclinical work for nuclear imaging trial?
Before you can start a nuclear imaging trial, there is certain preclinical work that must be completed. TRACER assists drug developers in both preclinical and clinical study activities. Below are our most requested preclinical services.
- Toxicity studies and evaluation of labeled compound
- Development of labeled antibodies, peptides, and small molecules
- Quality control and purification of labeled antibodies, peptides, and small molecules
- In vitro cell binding and internalization assay (Lindmo assay, IC50 assay, and Scatchard assay)
- In vitro autoradiography
- Immunohistochemical and Immunofluorescence staining
- Preparation and ethical approval of protocol for animal experiments
- In vivo biodistribution and PET and SPECT imaging in rodents
- Ex vivo autoradiography of radiolabeled compounds in excised organs
Read more about preclinical work before starting clinical trials.
Nuclear imaging in translational research
TRACER ensures a fast and seamless translation from preclinical to clinic. We recommend contacting us during your preclinical trajectory. Adhering to strict planning and clearly defined tasks is crucial to prevent duplication of work and can ultimately save valuable time and resources.
- GLP and GMP synthesis of precursors for labeling
- Design of radiolabeled compounds (and precursors)
- Radiolabeling of compounds for clinical use (GMP)
- Development, validation, and qualification of quality control assays
- Preclinical toxicity studies
- IMPD writing
- Manufacturing and release of labeled study drugs
What are the different types of nuclear imaging for drug development?
There are multiple medical imaging techniques, the four common ones are PET and SPECT (both nuclear) and MRI and CT (radiology). The biggest difference between these nuclear medicine imaging techniques is that PET and SPECT use a radiotracer that transmits radiation from within the subject while CT and MRI use external energy that is emitted from outside the body. For drug development, the two common methods of nuclear imaging are PET and SPECT. They do not provide anatomic information but visualize functions, biochemical changes, and molecular and cellular activity. All data that is vital to understand before taking the development of your compound to the next step.
Nuclear imaging clinical trial capabilities
TRACER designs and conducts clinical trials that provide accurate data on biodistribution and on-target and off-target accumulation using PET and SPECT imaging modalities. As a full-service imaging CRO our clinical services include:
- Clinical trial design for Phase 0, Phase I and Phase II clinical trials
- Statistical expertise for Phase 0, Phase I and Phase II clinical trials
- Preparation of clinical trial documentation for submission to authorities
- Interaction with regulatory authorities to receive clinical trial approval
- Preparation of Standard Operating Procedures (SOPs) for clinical trial conduction
- Preparation for data management, validation, and storage plan
- eCRF development
- Preparation of Trial Master File (TMF)
- Clinical study monitoring
- (Total body) PET/CT acquisition
- SPECT/CT acquisition
- Image analysis and dosimetry
- Patient recruitment & screening
- Data & image analysis, statistics, and reporting (Clinical Study Report)
Frequently asked questions about PET vs SPECT
PET and SPECT might seem similar, but there are a few key differences.
PET or SPECT study
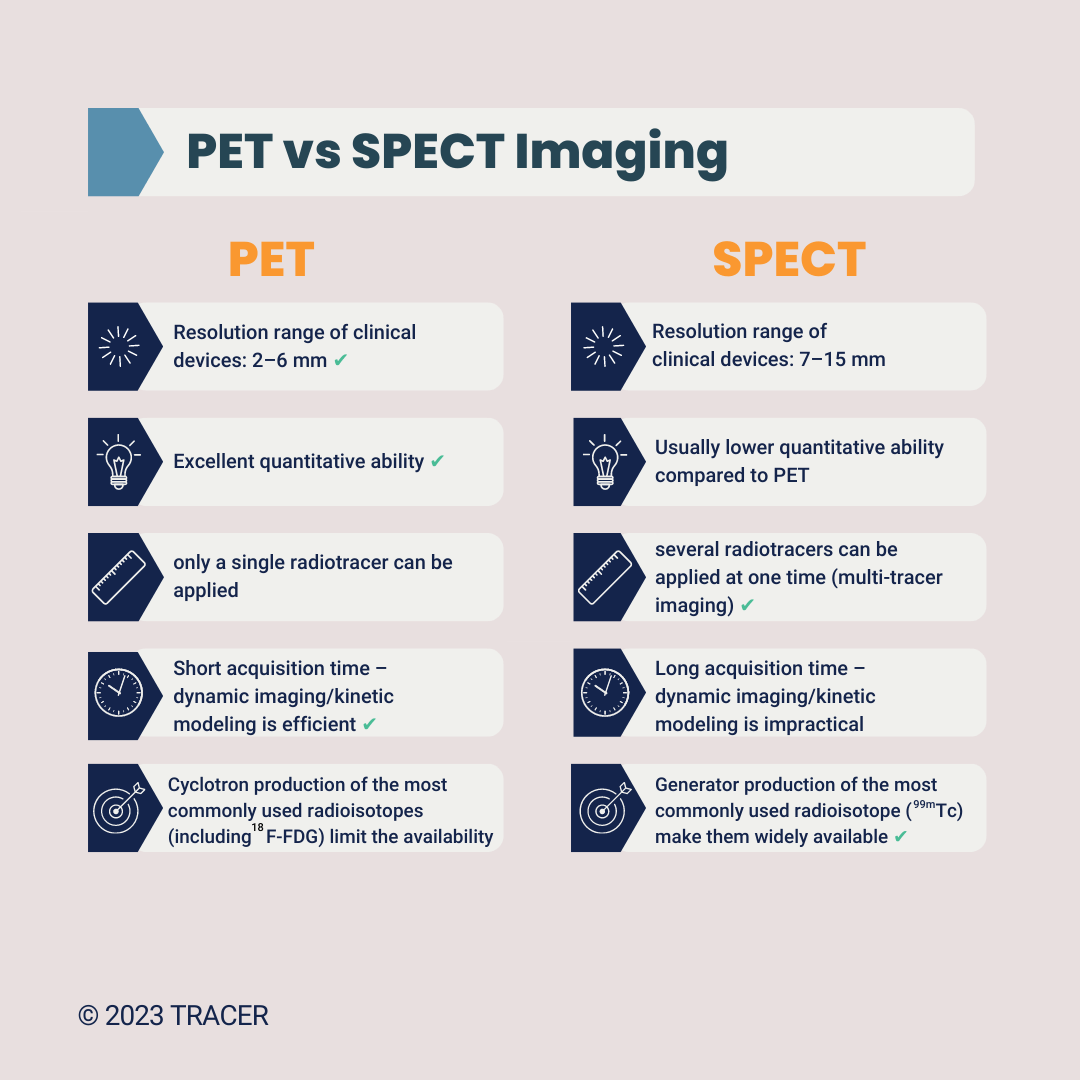
PET and SPECT are the two most used nuclear imaging techniques. They do differ in terms of resolution, sensitivity, quantification, availability, and costs. Because PET wins in terms of image quality and quantification, we mainly focus on that method. If availability and cost are important to you, we can discuss using SPECT instead of PET. To make the comparison yourself, we’ve written a comprehensive blog about SPECT vs PET. Because PET is often favorable, we use PET nuclear imaging examples on this page. However, feel free to ask us about SPECT clinical trials. In certain cases, a SPECT study can be the right solution.
What is PET?
Positron emission tomography (PET) is a medical imaging technique. As with other nuclear imaging modalities, PET uses low doses of radioactive substances to visualize biological processes in vivo. These radioactive substances can be referred to as tracers, or radiotracers. In a body, tracers will accumulate in areas with a higher degree of biochemical activity or higher receptor or transporter expression. Often a higher level of these processes coincides with the area of disease.
In early-stage drug discovery, research is done to identify these receptors. When the receptors or antigens have been identified, a compound that can bind to these antigens can be developed. At TRACER we are happy to share our knowledge on the many binding characteristics that we have experience with. Contact us to schedule a meeting.
Higher resolution imaging technique meaning a higher level of radiation?
In general, PET provides higher resolution nuclear images with a lower radiation dose than SPECT, but there are exceptions. The level of radiation is dependent on the dose administered to the patient, the energy of the gamma photons and the time the radionuclide is in the body. In this way, a SPECT bone scan can have a lower level of radiation than a PET bone scan. Fluorine-18 (F-18) labeled sodium fluoride (NaF) can be used for PET skeletal targeting. A Technetium-99m (Tc-99m) labeled bisphosphonates, such as methylene diphosphonate (MDP) used in SPECT, results generally in lower radiation exposure. Therefore, this SPECT method is currently still more commonly used than NaF PET.
The advantages of PET in drug development during early stages
Besides saving on costs and reducing the time to market, using PET in drug development has many other great advantages. The main advantage of PET is its very high sensitivity. This means very low concentrations of a radiopharmaceutical can be detected. As a result, low doses can be administered resulting in no or a very low biological effect of the radiopharmaceutical and low damage caused by the radiation. This way, biological processes in the body can be studied without saturating the molecular process. In a Phase 0 imaging clinical trial, only a microdose (subtherapeutic dose) is used. When using PET, a low dose can still result in very clear images.
Identify areas with a high risk of side effects early in the drug development process
A PET scan produces 3D images of the whole body and indicates the areas where the tracers have accumulated. In other words, a PET scan shows the distribution of the radiopharmaceutical throughout the whole body. When combining PET CT physiological and anatomical images can be created. By imaging the distribution of the radiopharmaceutical throughout the body, in addition to on-target accumulation the off-target areas can be identified where adverse effects might occur. This knowledge will benefit you in later clinical stages.
Go/No-Go decision
When developing new medicines pharmaceuticals, you want to acquire on-target and off-target quantification data as early as possible. This allows you to alter the compound if needed for late-stage clinical trials. An early Go/No-Go decision can save money and time and can allocate funds to pharmaceutical R&D instead of getting lost on failed clinical trials.
Use PET scanning for measuring the quantification of a drug in later clinical stages
PET is intrinsically quantitative. This allows for accurate quantification of concentrations of the radiopharmaceuticals in the body. Quantification of the radiopharmaceutical concentration grants valuable data. In later clinical stages, you can use PET to monitor treatment response with pre- and post-treatment measurements and sequential measurement of radiopharmaceutical concentration. Or for patient stratification.
Nuclear medicine scan to follow drug distribution over time
The good temporal resolution of PET allows for “dynamic imaging”. With dynamic imaging, the distribution of the radiopharmaceutical can be followed over time. A dynamic PET scan starts at the moment of the injection of the radiopharmaceutical and generally lasts an hour.
Afterwards, images of various time frames are reconstructed to visualize the distribution over time. This provides additional information on the metabolic processes in tissues of interest. Depending on the half-life of the chosen radionuclide, over the course of hours to days at certain time points a static scan can be made. This window should match the measuring period of the compound.
An industry example
What is an example of molecular imaging? One of the most well-known examples of PET is a tracer made out of glucose labeled with the positron emitter fluorine-18 ([18F]FDG). This tracer is used for the detection of tumors and their metastases. In the case of [18F]FDG, the substance is administered to the patient via an intravenous (IV) injection. In other words, IV directly injects the tracer into the vein. The radioactive glucose analog accumulates in cells that have a high glucose consumption, such as tumors. The [18F]FDG that is not consumed by the tissues in the body is cleared by renal excretion in the urine. After this biological process, the PET scanner can detect the gamma ray pairs emitted from the [18F]FDG accumulated in the tissues. Thus, the creation of a 3D image of where the tracers have accumulated is made possible.
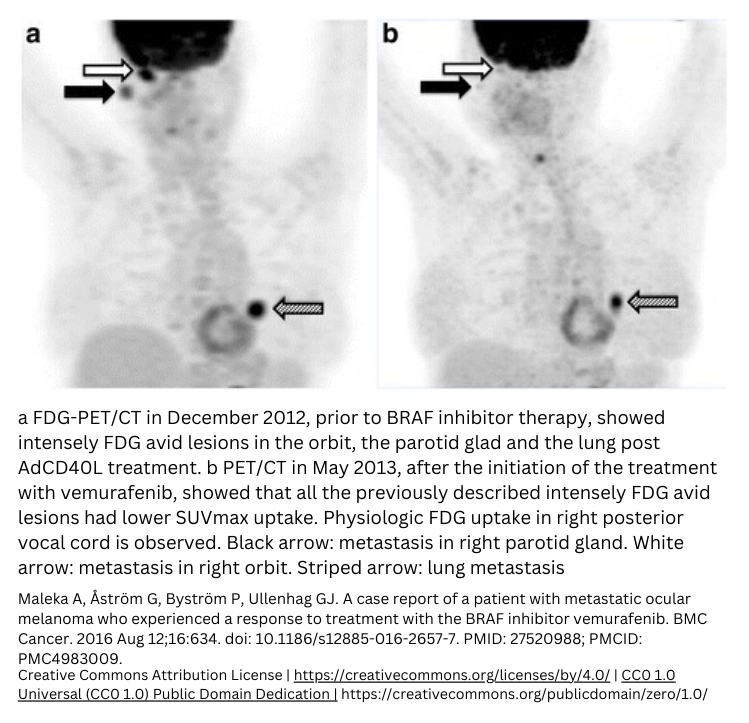
Molecular imaging examples
You can experience the power of combining molecular imaging and biology by looking at these molecular imaging examples.
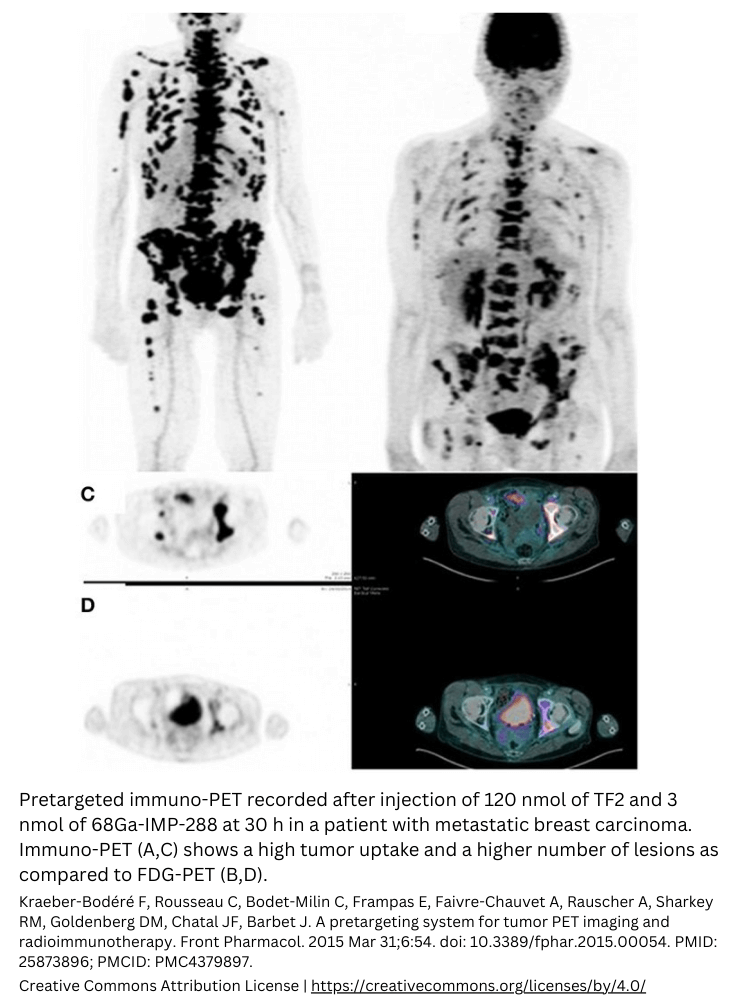
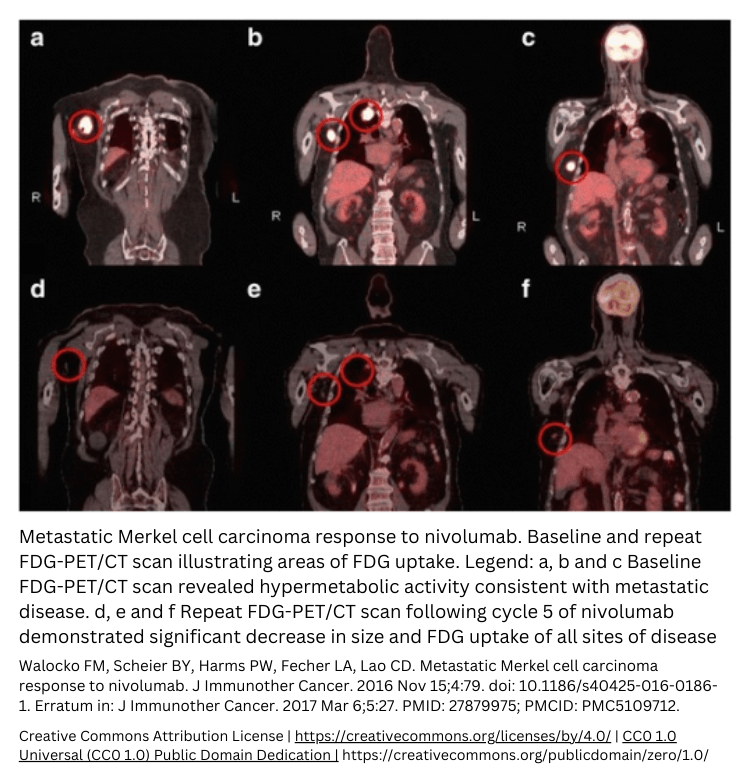
Radiopharmaceuticals in clinical trials
Radiopharmaceuticals in clinical trials give drug developers a whole new method to study their investigational new drug in vivo. Even before Phase 1. Radiopharmaceutical clinical trials are always conducted in a clinical trial center with experienced radiologists. As a drug developer, you don’t have to learn about radiotracers or radiopharmaceuticals; the whole labeling process and managing the study is done by TRACER.
Can a radiopharmaceutical used in nuclear medicine studies affect the outcome?
In theory, the radioisotope used for labeling can affect the outcome of the study. But by choosing the right tracer the behavior of your drug will be equal to the unlabeled compound. There are many types of nuclear medicines for nuclear molecular imaging. For each compound and target, one of the tracers is the best match for labeling without changing binding properties. TRACER is highly experienced in nuclear medicine and molecular imaging. When you want to know if a nuclear clinical trial is possible in your drug development, schedule a meeting with one of our scientists.
Schedule a meeting or send an e-mail to info@tracercro.com.
About TRACER – nuclear imaging CRO
Nuclear molecular imaging for drug development is a specialization that neither clinical trial companies nor molecular imaging companies by themselves offer. TRACER is a unique clinical research organization that was founded on the principle of adding nuclear imaging to clinical trials. We are a full-service clinical research organization with a strong focus on initiating and managing Phase 0 imaging clinical trials. Since 2006 the FDA has approved and actively encouraged drug developers to use Phase 0 clinical trials in their drug development. They want you to use it so you can identify the most promising compounds in your drug discovery pipeline and bring them to market as soon as possible.
What clinical trial benefits does TRACER offer?
The clinical trial services TRACER offers drug developers all share the same goals.
- Speed up the drug development process.
- Increase chances of success in subsequent clinical trials.
- Reduce costs in drug development that pharma can then invest in their drug discovery or the rest of their pipeline.
- Provide insights that are only available from molecular imaging and that can be used as a proof of concept.
- Help drug developers attract funding for their live-changing investigational new drugs.
Contact TRACER today to discuss the possibilities of nuclear imaging for your drug development. Or continue reading for answers to commonly asked questions about this subject.
Why conduct a nuclear medicine study with TRACER?
Setting up a nuclear medicine test for an investigational new drug requires experience. There is a (radio) chemical aspect and clinical aspect of conducting such a clinical trial. When using radiopharmaceuticals in nuclear pharmacy and nuclear medicine a compound needs to be conjugated with a label. There is also the imaging part of nuclear molecular imaging. If done right, an imaging clinical trial provides valuable data on which your future drug development relies and benefits from. So why conduct a nuclear medicine study with TRACER?
- The founder of TRACER, prof. dr. G.M. van Dam is a pioneer in molecular imaging.
- Scientists at TRACER are highly experienced in nuclear imaging.
- TRACER is the only clinical research organization that provides nuclear imaging at this level.
- Because TRACER conducts clinical trials in the Netherlands, pharmaceutical companies benefit from fast regulatory approval times and hig accrual rates.
- The method that TRACER uses is approved by the FDA and EMA.
Still not sure if nuclear molecular imaging can benefit your drug development process? Contact us to gain insights from case studies.
Is nuclear imaging the same as MRI?
No, nuclear imaging differs from Magnetic Resonance Imaging (MRI) in its core mechanism.
Nuclear imaging uses a radioactive medicine that emits radiation from the body allowing a special camera to visualize the location and quantity of the compound. Nuclear imaging is part of nuclear radiology, while MRI falls under radiology but is not nuclear. No radioactive radiation is released during an MRI scan.
MRI uses specific radiofrequency waves to produce images. On MRI images anatomy can be studied and abnormalities can be discovered. Functional processes cannot be seen on MRI, while this is possible with SPECT or PET images. An example that makes this perfectly clear; it is possible to detect cancer at an earlier stage with PET/SPECT than with MRI or CT.
Source: https://jnm.snmjournals.org/content/48/1_suppl/28S and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3075495/
Is it the same: molecular imaging radiology?
Are molecular imaging and radiology the same? No, molecular imaging originated from radiology but differs from it. Molecular imaging combines molecular biology and in vivo imaging. Molecular imaging is a form of functional imaging since processes in the body are visualized. Molecular imaging / functional imaging has the goal to visualize processes in the body. Molecular imaging allows studying metabolism, blood flow, chemical processes, absorption, and more. Instead of just visualizing what can be seen by using X-ray or frequency waves, it uses biomarkers as targets. Using biomarkers and binding molecules to them to create images, sets molecular imaging apart from radiology.
What is the main benefit of molecular imaging?
What was the greatest benefit the medical and research industry gained when molecular imaging was discovered in the mid-twenties of the previous century? For medical purposes, molecular imaging allows detection and diagnosis of diseases at a very early phase. This is possible since it can detect changes in chemical processes that happen before visible anatomical abnormalities occur. For drug development and drug discovery the benefits are even greater.
What are the benefits of molecular imaging in clinical trials?
Biodistribution and pharmacokinetics can be visualized in a clinical trial by using imaging. This is especially beneficial when molecular imaging is used in Phase 0. This first in human study allows drug developers to conduct a proof-of-concept (PoC) clinical trial at a very early stage in the drug development process. The PoC study is relatively inexpensive and can help with attracting the needed investment for the more expensive clinical trials that follow Phase 0.
How is molecular imaging used in clinical trials?
For a Phase 0 imaging trial, a microdose of the investigational new drug labeled with a radionuclide is administered to the clinical trial participants. Molecular imaging visualizes on-target and off-target accumulation of the compound in the body. This allows researchers to determine whether the compound may be successful in subsequent clinical trials based on efficacy data. TRACER specializes in clinical research imaging with nuclear or fluorescent tracers.
What is the difference between a CT scan and a nuclear scan?
A CT scan is very different from a nuclear scan based on how it is produced and what can be seen in the image. CT stands for Computed Tomography, and this definition is for ‘CT’ in SPECT the same. Sharing these letters does not mean that SPECT and CT are similar. CT and SPECT/PET are different based on the fact that the nuclear scan in SPECT/PET is constructed from radiation coming from within the body while a CT scan emits radiation externally through the body.
How does a CT scanner work and what is the difference between CT and X-ray?
The difference between a conventional X-ray scanner and a CT scanner is that the X-ray tube in a CT scanner rotates during the scan. On the opposite angle of the transmitting X-ray source, a detector picks up the signal and transfers it to a computer. This makes the creation of 3D images from a CT-scan possible instead of 2D images (X-ray). The computer then constructs the images. On every rotation, an image of a slice between 1-10 mm is made.
The generated 3D image shows the skeleton, organs, and tissue. In general, the skeleton is very well visible while for some tissues a contrast agent is needed to form a clear image. Contrast agents are not radioactive. On the contrary, commonly used contrast agents like Iodine-based and barium-sulfate compounds block radioactive material and therefore allow the CT scan to make a better image.
Is molecular imaging the same as nuclear medicine?
Molecular imaging and nuclear medicine overlap but are not the same. Molecular imaging uses nuclear medicine to study the physiological processes in cells. In molecular imaging these nuclear medicines are called radiopharmaceuticals, coming from the words radioactive and pharmaceuticals. These radioactive pharmaceuticals are purely used in molecular imaging to form an image of what is happening at a molecular level. The smallest amount of radiation is used to minimize the risk to the patient.
On the other hand, nuclear medicines can also have a therapeutic effect. The nuclear effect is then intentionally used to damage cells by exposing them to radiation. You can see nuclear medicine examples in radiation therapy for cancer, like bone, liver, thyroid, and prostate cancer, neuroendocrine tumors, and non-Hodgkin lymphoma.
Is molecular imaging by definition nuclear?
Molecular imaging can be done using nuclear medicine as in PET and SPECT. However, molecular imaging can also be done with other labels, tracers, or dyes. These can be fluorescent dyes or contrast agents for MRI and ultrasound. As long as a molecule is labeled for imaging, it can be seen as a method for molecular imaging. What method works for a specific application or study, depends on many factors.
Some things to keep in mind:
- A whole-body scan is often done with PET/SPECT.
- Uptake and distribution over time can be measured with a radioactive label.
- Fluorescence imaging has a very limited penetration depth depending on the tissue and is at the maximum around 3 centimeters.
- With MRI and CT imaging of anatomic structures is possible, but physiological processes cannot be seen.
- Function MRI (fMRI) and Molecular MRI (mMRI) are used in molecular imaging.
- Ultrasound can also be used in molecular imaging, it can provide data on types of tissue, blood flow, and motion.
- Molecular ultrasound involves imaging and even therapeutic uses of ultrasound by using contrast agents and biomarkers.
Why would you have a nuclear scan?
Nuclear scans allow us to visualize processes in the body. They are non-invasive, painless, and generally considered safe. Nuclear scans are used for diagnosis and therapy. For drug development nuclear scans visualize the biodistribution and pharmacokinetics of the investigational new drug (IND). When you participate in imaging clinical trials, the images are taken with PET or SPECT, and you are exposed to a small amount of radiation. Read the next question to learn more about this.
What are the suggested precautions after nuclear scan?
It is important to know that not every nuclear scan exposes the clinical trial participant or patient to the same amount of radiation. The amount of radiation can differ per type of radiopharmaceutical. It depends on the half-life of the radiotracer and the time between intake and excretion from the body. Here are several precautions after a nuclear scan or after the intake of a radiopharmaceutical.
– First of all, it is important to avoid contact with babies, children, and pregnant women for a period of several days.
– When participating in a clinical study where multiple scans are taken over a period of time, you can be asked not to drink too much since liquids help to flush the radiopharmaceutical out of the body.
Are there side effects of nuclear medicine?
Nuclear medicine exposes a participant in a clinical trial or a patient undergoing a PET or SPECT scan for diagnostic purposes to a very small amount of radiation. Nuclear medicines with a therapeutic effect expose the patient to a higher amount of radiation, as in the case of cancer radiation therapy. For a nuclear medicine scan, there can be side effects of nuclear medicine injection although they are not common. In general, the feeling of the radioactive injection for scan can be compared to that of a vaccination. When participating in a clinical trial, especially in a Phase 0 imaging clinical trial, the dosing is low/non-therapeutic.
What are the different meanings of nuclear?
In nuclear medicine and molecular imaging, the term nuclear refers to the use of radioactivity to create images from the radiation they emit. Nuclear can however have another meaning. Nucleus can refer in biology and chemistry to the central part of an atom. For instance, DNA is stored in the nucleus of a cell. In medicine, you find terms such as mononuclear infusion and mononuclear antibody. The first refers to the infusion of mononuclear cells. The second to monoclonal antibodies working together with mononuclear cells as in antibody-dependent cellular cytotoxicity ADCC.
Above were a few of the most common questions on the topic of nuclear imaging. want to know more about a specific question, or is your question not listed here? Then use the form on our contact page or send an email to info@tracercro.com.
Read more about molecular imaging
Nucl Med Mol imaging is short for Nuclear Medicine and Molecular Imaging, it is the journal of the Korean Society of Nuclear Medicine (KSNM). For more general information on molecular imaging, you can also visit the website of the Society of Nuclear Medicine and Molecular Imaging (SNMMI).