Oral drug delivery may be a preferred method because of ease of use or for targeted delivery to the gut. However, in drug development, oral administration poses new challenges. To name a few, the gastrointestinal tract (GIT) is a hostile environment, posing a risk of drug degradation. The mucosal barrier affects absorption into the bloodstream. Therefore, oral drug administration will always have lower bioavailability compared to systemic administration. How to understand the effect of the GIT on bioavailability, distribution, and pharmacokinetics? Assess the behavior of a new drug with Phase 0 exploratory trials and with nuclear imaging. TRACER specializes in this.
Are phase 0 exploratory studies suitable for oral drug administration?
Can phase 0 exploratory studies be used to assess the bioavailability, distribution, and pharmacokinetics (PK) after oral drug administration? The answer is yes, even in a microdose study. Highly sensitive analytical methods can detect very small concentrations of radioactive substances. Commonly used methods are positron emission tomography (PET), accelerated mass spectrometry (AMS), or liquid chromatography-tandem mass spectrometry (LC-MS/MS) [1, 2]. Depending on the chosen method, you can obtain different types of data.
Methods in microdose clinical trials for oral drug delivery
There are several highly sensitive methods commonly used in microdosing trials. Often microdosing clinical trials involve systemic administration, but these methods can also be applied to oral drug delivery.
Whole-body and total-body PET imaging
With whole-body and total-body PET imaging, biodistribution and PK data can be obtained, also in organs and tissues. For this, the drug needs to be labeled with a radionuclide. Not only can accumulation be visualized with a scan, but also the drug concentration in target tissue and organs can be calculated based on quantitative analysis. When scanning at multiple timepoints, the area under the curve (AUC)—concentration versus time after dosage—can be assessed and provides information on the residence time of the labeled drug. This data can also be used for a dosimetry study to calculate the absorbed dose to the subject and organs/tissues. PET is highly sensitive and especially useful in microdosing trials [3, 4].
AMS and LC-MS/MS for oral microdosing studies
AMS and LC-MS/MS can be used in microdosing studies. These methods don’t offer a visual image like PET, but only measure the concentrations of drugs in body fluids and tissue biopsies. For AMS analysis, compounds must be labeled, often with carbon-14 (14C). LC-MS/MS can be used with unlabeled compounds when concentrations are sufficient (which is not always the case in microdosing studies). [7, 8] Again, for comparison, neither of these methods uses imaging and therefore doesn’t show accumulation and distribution in tissues and organs.
Is oral administration of PET tracers possible?
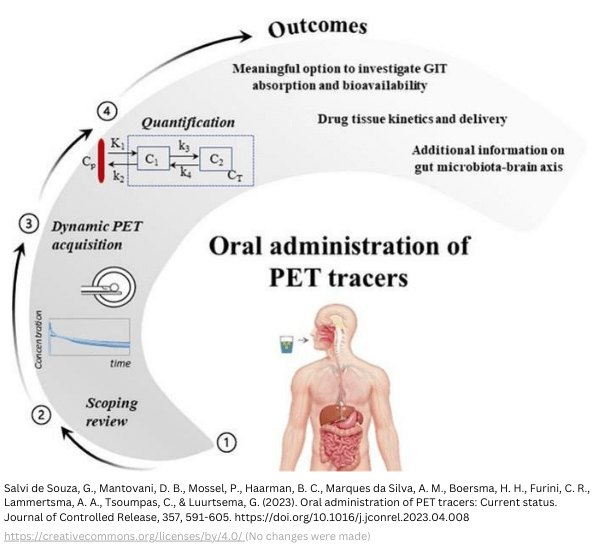
For those who remember that PET imaging was not possible for oral drug administration. Nowadays it is, thanks to total-body and whole-body PET imaging. Traditionally, oral administration was unsuitable for radiolabeled drugs due to the high radiation exposure of organs. Although with conventional PET scanners, oral PET has been reported [3]. Oral administration is possible with low doses, a characteristic of Long Axial Field of View (LAFOV) scanners, also known as whole-body PET scanners (axial field of view (aFOV) around 100 cm). When the aFOV is around 200 cm, ultra-extended, it is called total-body PET. These scanners have much better sensitivity and drastically decrease the radioactive dose required for high-resolution images [5].
Influencing factors in oral drug delivery
Oral drug delivery has influential factors from the GIT that should be assessed in clinical trials.
- Oral drug delivery may require administration of an additional amount to compensate for first-pass metabolism.
- Research into absorption, distribution, metabolism, and excretion (ADME) and characterization of pharmacokinetics are important when choosing oral drug administration.
- In addition, food effect studies should be conducted to study the influence of metabolism with food intake versus fasting and to compare the effect of different types of food.
- There may be differences between participants.
Patient variability
In addition to interactions between drugs and foods, special attention must also be given to variability between patients. This variability is due to the patient’s gender and age (e.g., liver function), anatomical, physiological, and genetic variables (e.g., pH, digestive enzymes, microbiome, surface areas, thickness and permeability of the mucous layer, and peristaltic movement), individual diets and use of other drugs (food-effect and drug interactions), and adherence to dosing schedules. PET imaging can be a useful tool for understanding bioavailability, distribution, and absorption, as well as variability between patients.
Why are exploratory trials for oral drugs so important?
TRACER specializes in exploratory trials with PET imaging. Here are our three main reasons why Phase 0 exploratory trials for orally administered drugs are so important.
- The best model for humans is human. ADME in humans can differ greatly from that in animals due to differences in the GIT.
- Drug bioavailability is greatly influenced by the GIT and can vary from patient to patient.
- Microdosing clinical trials reduce the risk for participants and provide data to inform the Phase 1 starting dose.
Methods for imaging of oral drugs
When choosing imaging for your clinical trial, you need to know that there are multiple methods available. But what method(s) to choose?
PET, SPECT, Gamma-scintigraphy, MRI and CT
PET imaging is commonly used and often preferred due to its high resolution and sensitivity. What are the other methods, and what are their drawbacks compared to PET?
- Single-photon emission computed tomography (SPECT) uses gamma (γ) emitting radionuclides to create 3D images. It often produces lower resolution images than PET.
→ To learn more, read our full PET vs SPECT comparison. - Gamma-scintigraphy also uses γ-emitters, but produces planar images (2D).
- Magnetic resonance imaging (MRI) does not use radiation but radio waves and magnetic fields. It is commonly used for anatomical analysis (soft tissue, e.g. brain, muscles, organs) and not functional analysis. Functional MRI (fMRI) is an exception that uses contrast agents to obtain functional information, although it cannot be compared to PET or SPECT.
- Computerized tomography (CT) uses X-rays, but, similar to MRI, is used to show anatomical structures (e.g. bone, brain, organs, arteries and blood vessels) and not functions.
- Often, a combination of PET or SPECT for function and MRI or CT for anatomical reference is used.
Considerations for oral PET imaging
PET imaging of orally administered drugs is still a relatively new field and therefore lacks the strong foundation of parenterally administered drugs. Here are some considerations for your imaging strategy:
- PET radionuclides already used for oral administration include, Fluorine-18 (18F), Copper-64, (64Cu) [9], Zirconium-89 (89Zr), Carbon-11 (11C) [5], Iodine-124 (124I) [10].
- Gamma emitting radionuclides (for SPECT or gamma scintigraphy) already used for oral administration include Indium-111 (111In), Technetium-99m (99mTc), and Samarium-153 (153Sm (from neutron activation of 152Sm)) [11,12], Iodine (such as 131I or 123I) [13].
Note: the list above includes clinical or preclinical used radionuclides in various tracer formulations. Over time, new radionuclides may enter the clinical field.
Imaging GIT function and gastrointestinal drugs
Oral administration can be used for systemic drugs, but is often the preferred route for gastrointestinal drugs; medication to treat gastrointestinal diseases. This different starting point changes the requirements for drug formulation and imaging.
- For imaging of the GIT itself, you need to use a non-absorbable formulation or a formulation that gets activated at the target site.
- For tracing a drug in the GIT, you need a formulation that survives the harsh environment of the GIT. When activating at a specific site, you need to include this in your imaging strategy.
- Choosing a radionuclide with an appropriate half-life should take into account that complete digestion may take up to 72 hours.
Note: in all cases, the labeling should not interfere with drug behavior.
Suggestions from other oral drug imaging studies
Here are some valuable suggestions from other oral drug imaging studies that may be useful when designing your clinical trials.
Quantification of data from microdosing studies
Multiple studies have verified the reliability —defined as within 2-fold of the actual human pharmacologic dose PK— of quantification from a microdose to therapeutic levels. As could be expected, i.v. administration always results in a higher predictability compared to oral drug administration. Drug concentration levels in organs and tissues can be calculated from quantitative analysis of PET and SPECT images. More often, concentrations are measured by AMS in biofluids.
- In this study, 41 orally administered drugs showed a 68% predictability against 94% from 16 i.v. administered drug [17].
- In another study of 35 compounds, 79% proved accurate scalability for oral, and 100% for i.v. administered drugs [18, 19].
Microdose vs other methods of predicting human pharmacokinetics
Compared to other methods, such as allometry (widely used), physiological-based pharmacokinetic (PB-PK) modeling, and in vitro–in vivo extrapolation (IVIVE), microdosing does not underperform. On the contrary, the accuracy to predict human pharmacokinetics for these methods (within a factor of two) for oral administration has been reported to be 45% at best [20, 21]. In another paper on the accuracy of PK prediction for AstraZeneca, from 97 compounds, 60 were considered successful (within twofold), and 37 were unsuccessful (>5-fold from observed for one or more parameters) [22]. Nonlinear disposition from microdose to therapeutic dose are often the result of saturable processes [23].
Conclusion
By incorporating Phase 0 microdosing studies in your drug development program, a better understanding of ADME after oral administration can be established. Resulting in:
- Biodistribution and pharmacokinetics assessment in patients at the earliest possible time.
- Smarter subsequent clinical trial designs based on human data.
- Identify the need to compare populations (read also: bridging studies).
- Develop patient-specific treatment.
- A comparison between oral and i.v. with a crossover study design [24].
TRACER specializes in Phase 0 and imaging trials
Would you like to learn more about the possibilities of clinical trials for orally administered drugs? TRACER, a contract research organization (CRO) for early phase clinical trials, can assist you with this. We are happy to share our knowledge and discuss how Phase 0 and (PET) imaging can be used for your drug development program. Make an appointment using the button below.
Frequently asked questions
Since oral delivery in imaging trials is relatively new, we often get questions on this topic. Below, we answer some of these questions. If you have another question, simply use the contact form to obtain answers.
What is needed for imaging trials: a drug product or drug substance?
For imaging trials with oral delivery—as well as i.v.—, we need a drug substance, not yet a finalized product. The drug substance will be labeled for imaging in clinical trials. The formulation should not be finalized yet. After formulation, it cannot be labeled. When you conduct a Phase 0 clinical trial, often a Good Laboratory Practice (GLP)-grade drug substance is sufficient. When the drug is labeled under Good Manufacturing Practice (GMP) conditions, the clinical trial material is considered GMP-grade and therefore suitable for human use.
Is oral administration in preclinical trials possible?
Yes, oral administration is possible in preclinical trials. TRACER specializes in in-human studies, but we also conduct the preclinical work needed to move to clinical.
What radionuclides and chelators are suitable for oral administration?
Earlier in the article, we discussed radionuclides currently used orally. This list includes Fluorine-18 (18F), Copper-64, (64Cu) [9], Zirconium-89 (89Zr), Carbon-11 (11C) [5], and Iodine-124 (124I)[10]. Preferably, the radionuclide replaces a non-radioactive atom in the molecular structure. This method is not always available, for example, labeling with 64Cu or 89Zr is only possible with a chelator. Chelators may alter the drug’s behaviour, although this can be done intentionally so the tracer won’t be absorbed during GIT imaging. When chelators are used while absorption is intended, the altered compounds should be compared with unaltered compounds in terms of chemical properties and stability. Some chelators are mentioned in the referenced papers. For advice on this, please contact us.
Can you give examples of PET scanners used for oral drug imaging?
LAFOV PET scanners are used for orally administered tracers to measure a drug’s biodistribution. At TRACER, we often use the whole-body Siemens Biograph Vision Quadra™ PET/CT Scanner. But also standard FOV scanners, like the Siemens ECAT EXACT HR47 have been reported [3] to be used. The chosen equipment often depends on what is available at the clinical site. A thorough inventory of equipment and standardization of data are important for multisite clinical trials. TRACER can help you with this. We have established partnerships with clinical sites.
What types of drugs can be labeled and imaged after oral administration?
Both small molecules and biologics, like antibodies, can be labeled for imaging. If administered orally, the imaging —and in case of biologics, surviving the GIT—may be more challenging. It mostly depends on the type of labeling, with chelator or without, and if the drug is intended for absorption or not. Generally speaking, for small molecules, there are more possibilities. For biologics, like antibodies, oral administration requires a different strategy, not only for imaging, but also for a formulation to deliver it to the target [25]. What this shows in all cases of oral administration, Phase 0 and PET imaging offer valuable data that contribute to the overall success.
Abbreviations
| ADME | Absorption, Distribution, Metabolism, and Excretion |
| aFOV | axial Field Of View |
| AMS | Accelerated Mass Spectrometry |
| AUC | Area Under the Curve |
| CRO | Contract Research Organization |
| CT | Computerized Tomography |
| fMRI | functional MRI |
| GIT | GastroIntestinal Tract |
| i.v. | intravenous |
| IVIVE | In Vitro–In Vivo Extrapolation |
| LAFOV | Long Axial Field of View |
| LC-MS/MS | liquid Chromatography-Tandem Mass Spectrometry |
| MRI | Magnetic Resonance Imaging |
| PB-PK | Physiological-Based PharmacoKinetic |
| PET | Positron Emission Tomography |
| PK | PharmacoKinetics |
| SPECT | Single-Photon Emission Computed Tomography |
Citations
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, or copyrighted terms wherein.