Central Nervous System (CNS) drug development has a unique challenge of its own when compared to non-CNS drugs. To reach the brain, it needs to cross the Blood Brain Barrier (BBB). To understand the current state of how that question is answered, we asked numerous professionals in the field two questions. What methods are currently used in CNS drug development to assess BBB crossing? And, what factors should they consider when incorporating (nuclear) imaging? In this blog, we cover their answers, discuss imaging for CNS drug development, and, last but not least, give you a list of tracers used for CNS imaging.
Download list of CNS tracers

What are the current methods used to measure drug delivery of CNS drugs to the brain in clinical trials?
What are the methods to evaluate CNS drugs without the use of imaging? These are the methods commonly mentioned by professionals in the field of CNS drug development.
- Human CerebroSpinal Fluid (CSF) sampling via lumbar punctions is considered to be the best method to study unbound-drug concentrations in the brain.
- Blood and plasma samples for measurements of drug concentration and to evaluate biomarkers.
- Non-invasive techniques like Quantitative ElectroEncephaloGraphy (QEEG) are used to assess brain activity.
- Behavior assessment to evaluate cognitive and motor functions.
- Microdialysis is mainly used in preclinical animal studies, but can also be applied in in-human studies.
What are the considerations for using (nuclear) imaging in clinical trials?
Nuclear imaging, such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT), is a relatively non-invasive method to visualize and quantify the biochemical and physiological processes within the CNS. This provides valuable data for developing CNS-targeted drugs. The recent development of many CNS tracers (>100) offers exciting new opportunities for imaging. Fill out your e-mail and we send you the list of CNS tracers for free.

How do drug developers look at imaging in CNS clinical trials?
What are the current considerations for using imaging in CNS clinical trials?
- Depending on the disease, imaging is more widely adopted, for example, in Alzheimer’s disease.
- Imaging is more commonly used for early phase clinical trials rather than for Phase II and III
- Important aspects when considering are the costs, expertise, and the needed infrastructure.
- Imaging is valuable for exploratory data to assess brain penetration, target engagement, and receptor occupancy, and can help substantiate hypotheses for compound selection and dose estimation.
- To standardize results in early-phase clinical trials, a larger sample size might be needed.
- For late-stage development, imaging endpoints are considered valuable when required for regulatory approval or included in the final drug label. This may be the case when imaging endpoints directly relate to efficacy and safety, and standard of care decisions.
- In a multi-center, multi-country study, achieving standardized results is more challenging due to variability between operating teams and logistical factors (e.g., time slots, tracer preparation, etc.)
- Imaging biomarkers are not available for all targets, either due to lack of development, ongoing development, or because the tracers are not commercially available.
Can we use imaging in Phase 1?
A question we often get at TRACER, imaging CRO, is the possibility of using imaging in Phase 1 drug development. For Phase 1, where safety and tolerability are assessed, this is generally done with an unlabeled and unaltered drug product. Since many CNS drugs are small molecules that should cross the BBB, it can be challenging to couple them to a radionuclide, as this may alter the molecule’s properties. However, imaging in Phase 1 clinical trials is still possible, even without labeling. Let’s compare labeling the drug versus the use of separate imaging biomarkers.
Labeling the compound or measuring imaging biomarkers
A tracer, necessary for imaging, can be used in two ways. One method is labeling, where a radionuclide is coupled to the compound with a linker. The coupled compound is then administered to the subject. In doing so, biodistribution (BD) and pharmacokinetics (PK) data can be generated. The labeling approach is typically used in a Phase 0 microdosing study. For instance, one of our sponsors in CNS drug development uses this method in exploratory research to assess the distribution of the active compound within the CNS – or even the whole body – and determine its residence time.
Imaging biomarkers for response monitoring
In addition to labeling the drug and then administering it to the subject, there is an alternative approach. Imaging biomarkers can be used for response monitoring in patients. The approach is as follows: First, the radiotracer is administered to measure the baseline response of the drug’s target. Then the drug is administered, and after a predefined time, the tracer is again administered to measure the effect of the therapy (Figure 1).
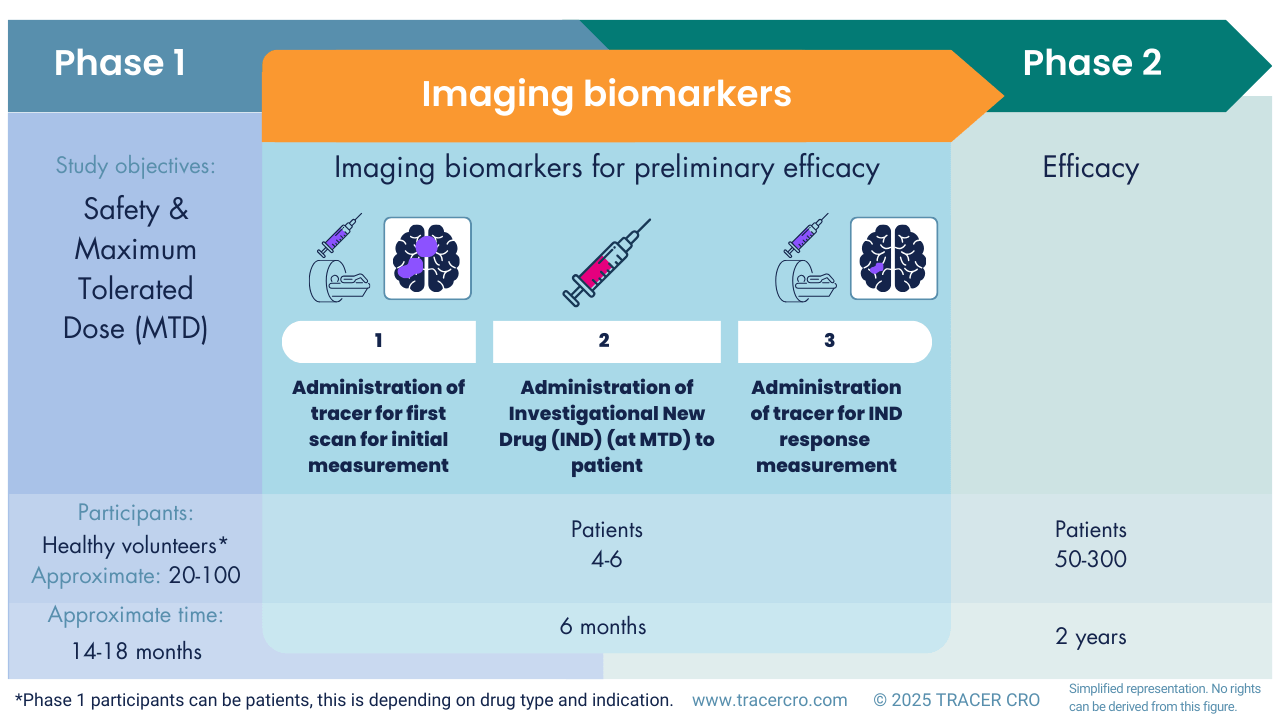
Fig 1. Showing how imaging biomarkers can fit between Phase 1 and 2 as a substudy.
Substudy of Phase 1
In a Phase 1 trial, an imaging study can be implemented as a substudy involving a small cohort of 4–6 patients. When the tracer is already commercially available, a substudy like this should not take more than approximately 6 months. This can be done in parallel to preparations for Phase 2. The imaging biomarker should be carefully chosen to align with the biological target of your investigational drug. The acquired data provides early insights into your drug’s efficacy and helps confirm that it reaches its intended target and successfully crosses the BBB, based on observed pharmacological effects.
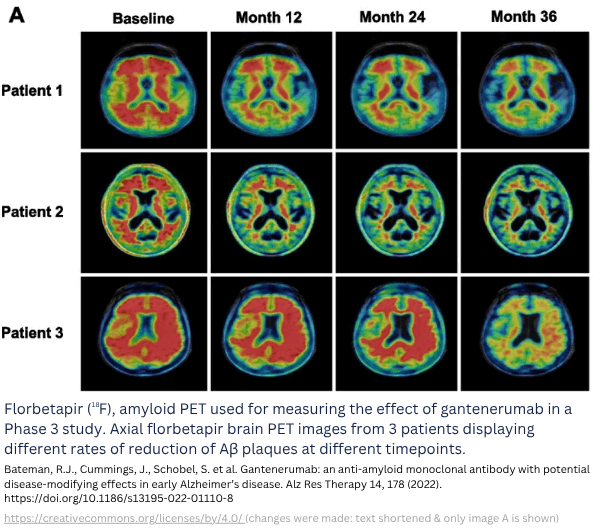
Fig. 2: Example of biomarker imaging to observe treatment response. Example of a study with imaging biomarkers [1] (study not conducted by TRACER).
Advantages and disadvantages of both imaging strategies
Labeling the drug with a tracer has some unique advantages:
- Data on full-body biodistribution is available in real-time.
- The effective dose in target and off-target tissue can be estimated via dosimetry calculations.
However, there are also some advantages of using imaging biomarkers:
- Integrating imaging biomarkers as a substudy in a Phase 1 trial saves time and costs compared to the labeling approach, which may take more time.
- Many tracers for CNS are commercially available, which means they are approved for in-human use and can be included in a clinical study easily.
- The imaging biomarker does not influence the behavior of the compound.
- Little to no additional Chemistry Manufacturing and Control (CMC) and regulatory steps are required when your compound has already been approved for the clinical trial, and the tracer used has approval for in-human use.
- By using this route, there’s no need to set up an entirely new trial; only a substantial amendment is needed for regulatory approval.
Discuss this and other possibilities with TRACER to learn what solution will give you the best data.
Contact TRACER
Imaging compared to non-imaging methods
Non-imaging methods, such as CSF fluid, provide data on the amount of drug in the CSF, but it remains unknown whether the drug actually reaches its target in the brain. In contrast, imaging offers detailed data on the disease and the drug’s mechanism of action. Especially in an early phase, imaging can be used for improved compound selection and a better understanding of the disease and the related compound’s PK.
Conclusion
Incorporating imaging biomarkers into a Phase 1 substudy provides early insights into your drug’s efficacy and helps confirm that it reaches its intended target. The imaging biomarker should be carefully selected to align with the biological target of your investigational drug. For some targets, suitable imaging biomarkers may not be available (yet). Compared to labeling, this method is faster and more economical.
Incorporating imaging in clinical trials requires specialized expertise and infrastructure, which may be seen as a hurdle. When working with a specialist like TRACER, you can quickly make use of the existing infrastructure and knowledge. Getting your imaging trial quickly of the ground.
Frequently asked questions
There are some frequently asked questions related to CNS drug development.
Can you give background information on the Blood Brain Barrier?
The BBB is a selectively permeable barrier formed by endothelial cells, tightly connected by junctions that make it difficult for large molecules or harmful substances to pass through. Its primary function is to protect the brain from potentially harmful substances in the bloodstream. However, this protective mechanism also limits the ability of drugs to reach specific areas of the brain, as many fail to cross the BBB. In certain neurological diseases, the BBB can become weakened or damaged, allowing harmful substances to enter the brain and potentially worsen the progression of the disease, while on the other hand, affecting the passing of the BBB by a drug.
What are examples of preclinical models in CNS drug research?
Various preclinical models are used to gather data on CNS drugs. Some examples of current approaches include brain organoid models, Blood-Brain Barrier-on-a-Chip, hCMEC/D3 cell line to simulate the human blood-brain barrier, and induced Pluripotent Stem Cells (iPSCs), among others. For example iPSC, can be used to model neurodegenerative diseases. Additionally, animal models are utilized, and compared to in-human studies, they allow for more invasive techniques, such as microdialysis and post-mortem sample collection, offering deeper insights into CNS pharmacology and drug effects that are not always feasible in humans.
What are the challenges of CNS drug development?
CNS drug development faces several significant challenges, with one of the most prominent being the difficulty of crossing the BBB to effectively deliver drugs to the brain. Another major hurdle is the translation of preclinical findings into clinical studies, as preclinical models offer more methods for obtaining brain data, while options are more limited in humans. Additionally, many CNS drug candidates fail due to a lack of efficacy, as well as variability caused by the heterogeneous nature of CNS diseases. Furthermore, the underlying pathophysiology of CNS disorders is often not fully understood and/or is multifactorial.
What does CNS mean in pharma? What are CNS drugs?
The CNS is responsible for processing and controlling most bodily functions. It consists of the brain and spinal cord. There is a broad range of diseases that affect the CNS. Examples include neurodegenerative diseases (such as Alzheimer’s, Parkinson’s, Amyotrophic Lateral Sclerosis (ALS)), autoimmune disorders, inflammatory conditions, cerebrovascular diseases, brain tumors, infections, mental health disorders, and more. These conditions can impact the brain, spinal cord, and associated neural structures. A variety of drugs have been approved for treating CNS-related conditions. In pharma, CNS drugs include anaesthetics, anticonvulsants, cognitive enhancers, antiparkinsonian agents, antidepressants, and others. These medications help manage symptoms and, in some cases, can modify the progression of CNS disorders.
Abbreviations
| CNS | Central Nervous System |
| BBB | Blood Brain Barrier |
| CSF | CerebroSpinal Fluid |
| QEEG | Quantitative ElectroEncephaloGraphy |
| PET | Positron Emission Tomography |
| SPECT | Single Photon Emission Computed Tomography |
| CRO | Contract Research Organisation |
| BD | Biodistribution |
| PK | Pharmacokinetics |
| CMC | Chemistry Manufacturing and Control |
| iPSC | induced Pluripotent Stem Cell |
| ALS | Amyotrophic Lateral Sclerosis |
Citations
Although this article has been composed with great care and attention, we cannot guarantee its accuracy. If you have any suggestions or additions to this article, please email info@tracercro.com.
No rights can be derived from this publication. This blog does not make claim or promote ownership to any intellectual property, study information, or copyrighted terms wherein.