How can imaging be integrated into Phase 1 studies, and even more challenging, how can this be done for CNS drug development? Our intern Anne Miggelenbrink did a deep dive; she talked with drug developers in the field and discussed possibilities with TRACER’s imaging experts. While Anne was busy with that, Carlijn Hut went on an internal investigation. She mapped TRACER’s processes and is writing her plan that could contribute to optimization. As you see, at TRACER, an intern is not doing a typical intern’s job – no coffee fetching, no archive filing — just meaningful work that contributes to real drug development.
Update Anne & Carlijn
In this blog, we’ll give an update on how it’s been so far for Anne and Carlijn to work on real projects that could have a real impact on the organization and even on drug development.
Integrate imaging into Phase 1 studies, Anne’s approach
To answer this question, one paragraph is way too short. So, we’ve written a whole blog on an unconventional method, not yet widely used in CNS drug development. Anne explains, “First I did market research, what methods in CNS drug development are used in Phase 1 to evaluate Blood Brain Barrier crossing? Knowing the current status, I could compare this to imaging and TRACER’s methods and see the potential benefits imaging could bring to drug development programs (but also some hurdles). Furthermore, I found out there are CNS-related tracers on the market, some already commercially available and others that can be used in a clinical research setting. I’ve added them to a list, complete with indications, that drug developers now get access to. They only have to download it to learn their options.”
Read the blog:
CNS drug development
Mapping the clinical study process: Carlijn’s insights
Before clinical research can be conducted, many different steps and activities need to take place, involving numerous people. This happens both internally, between different divisions of the company, and externally, with various stakeholders. Of course, there is the sponsor, but also ethical committees that need to approve the study, clinical sites where the study takes place, and the external monitor, who ensures everything follows the plan and guidelines. Carlijn, “I did a stakeholder analysis and conducted interviews with everyone involved in the process. In just a few months, I gained a clear understanding of the full process needed to actually test a new drug in patients.”
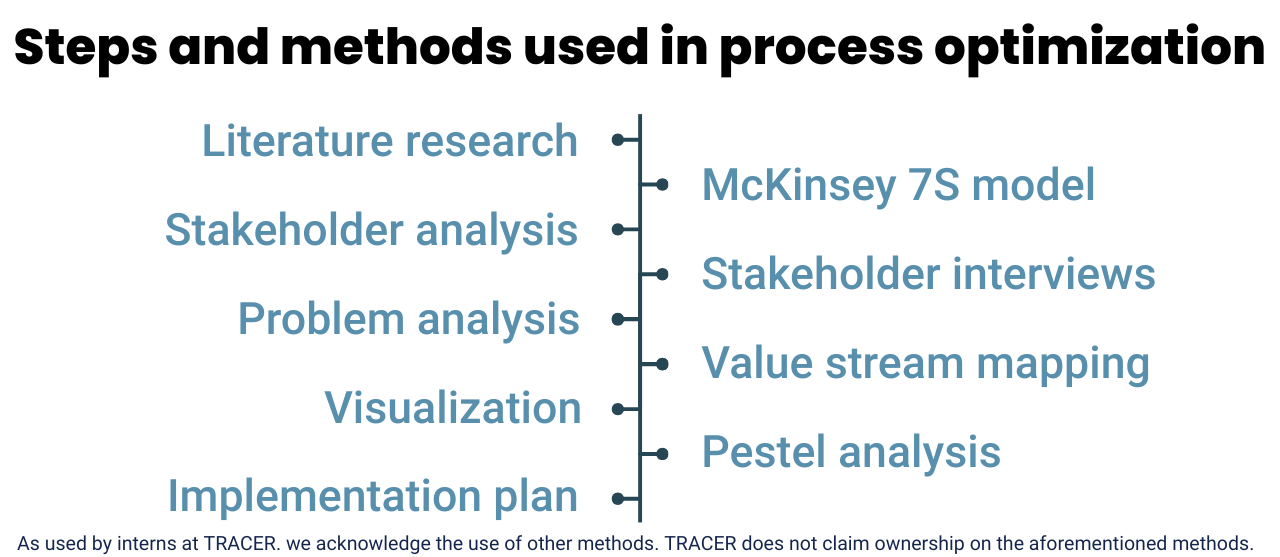
(Steps and methods Carlijn uses to understand current processes and gain insight into opportunities for optimization.)
Visiting the GMP facility
Anne and Carlijn visited one of our CMC/GMP manufacturing sites, where they had a tour of the various labs. Carlijn, “It was really interesting to be able to take a look around. It is very impressive to see how many measures have to be taken to work in such a lab. You think about it beforehand, but once you walk around, it is even more impressive seeing what can be done and that, ultimately, labeling for the patients’ doses is done right there. It then becomes really clear that in a clinical trial, many parties have to work together to get results.”
What’s next?
The internships are not over yet, even though from the amount of work done, you might expect that. What awaits you in the coming months?
Coming up: visualization of the standardized process
Carlijn, “Over the next few months, all the interviews are going to be processed, eventually resulting in a visualization of the standardized process. Furthermore, if there is still time, I will do a PESTEL analysis to see if this process is future-proof. it will also be interesting for me to see how my suggestions will lead to an improved process in practice.
Coming up: single and multi-site studies
Anne, “The question above was only my first research question. I’ll continue by investigating differences between single-site and multi-centre studies, examining their timelines, costs and value proposition. So, in a next blog I will keep you posted on those results.”

